 | |
| Names | |
|---|---|
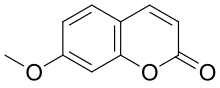
| Preferred IUPAC name 7-Methoxy-2H-1-benzopyran-2-one | |
| Other names
7-O-Methylumbelliferone 7-Methoxycoumarin Ayapanin Herniarine Methyl umbelliferyl ether | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.741 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H8O3 |
| Molar mass | 176.171 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Herniarin is a natural chemical compound. Chemically, it can be considered a methoxy derivative of coumarin or a methyl derivative of umbelliferone.
Herniarin is found in Herniaria glabra, Ayapana triplinervis and in species of the genus Prunus (P. mahaleb, P. pensylvanica, and P. maximowiczii).
References
- "Herniarin". liberherbarum.com.
- Santamour F. S. and Riedel L. G. H. (1994). "Distribution and inheritance of scopolin and herniarin in some Prunus species". Biochemical Systematics and Ecology. 22 (2): 197–201. Bibcode:1994BioSE..22..197S. doi:10.1016/0305-1978(94)90008-6.
| Types of coumarins | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||
| Glycosides | |||||||||||
| Derivatives |
| ||||||||||
| Oligomers | |||||||||||
| Synthetic | |||||||||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |