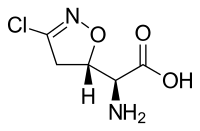
 | |
| Names | |
|---|---|
| IUPAC name (2S)-Aminoethanoic acid | |
| Other names Antibiotic AT 125 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H7ClN2O3 |
| Molar mass | 178.574 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Acivicin is an analog of glutamine. It is an inhibitor of gamma-glutamyl transferase.
It is a fermentation product of Streptomyces sviceus. It interferes with glutamate metabolism and inhibits glutamate dependent synthesis of enzymes, and is thereby potentially helpful in treatment of solid tumors.
After its discovery in 1972, acivicin was studied as an anti-cancer agent, but trials were unsuccessful due to toxicity.
Research
An in vitro study showed that Acivicin at a concentration of 5 μM Acivicin inhibited by 78% the growth of human pancreatic carcinoma cells (MIA PaCa-2) after 72 hours in continuous culture. It was also found that acivicin at a concentration of 450 μM irreversibly inactivated MIA PaCa-2 γ-glutamyl transpeptidase (10 nmol/min/10 cells) with an inactivation half-life of 80 minutes.
Phase I studies
Phase I dose escalating studies conducted in 23 cancer patients administered acivicin with a concomitant 96-h i.v. infusion of a mixture of 16 amino acids showed reversible, dose-limiting CNS toxicity, characterized by lethargy, confusion and decreased mental status.
References
- ^ Allen, L.; Meck, R.; Yunis, A. (1980). "The Inhibition of γ-Glutamyl Transpeptidase from Human Pancreatic Carcinoma Cells by (αS,5S)-α-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid (AT-125; NSC-163501)". Research Communications in Chemical Pathology and Pharmacology. 27 (1): 175–182. PMID 6102405.
- Hidalgo, M.; Rodriguez, G.; Kuhn, J. G.; Brown, T.; Weiss, G.; MacGovren, J. P.; von Hoff, D. D.; Rowinsky, E. K. (1998). "A Phase I and Pharmacological Study of the Glutamine Antagonist Acivicin with the Amino Acid Solution Aminosyn in Patients with Advanced Solid Malignancies". Clinical Cancer Research. 4 (11): 2763–2770. PMID 9829740.
- Kreuzer, Johannes; Bach, Nina C.; Forler, Daniel; Sieber, Stephan A. (2015). "Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition". Chemical Science. 6 (1): 237–245. doi:10.1039/C4SC02339K. PMC 4285139. PMID 25580214.
External links
- Obrador, E.; Carretero, J.; Ortega, A.; Medina, I.; Rodilla, V.; Pellicer, J. A.; Estrela, J. M. (2002). "γ-Glutamyl Transpeptidase Overexpression Increases Metastatic Growth of B16 Melanoma Cells in the Mouse Liver". Hepatology. 35 (1): 74–81. doi:10.1053/jhep.2002.30277. PMID 11786961.
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R. M.; Gerhard, M. (2007). "Inhibition of T-Cell Proliferation by Helicobacter pylori γ-Glutamyl Transpeptidase". Gastroenterology. 132 (5): 1820–1833. doi:10.1053/j.gastro.2007.02.031. PMID 17484877.
| Leukotriene signaling modulators | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||
| Others | |||||||||||||||||
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |