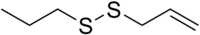| Names | |
|---|---|
| Preferred IUPAC name 3-(Propyldisulfanyl)prop-1-ene | |
| Other names
2-Propenyl propyl disulphide 4,5-Dithia-1-octene Onion oil 2-Propenyl propyl disulfide Propyl allyl disulfide 1-Allyl-2-propyldisulfane (not recommended) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.016.864 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1993 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H12S2 |
| Appearance | Pale-yellow liquid |
| Odor | strong onion-like odor |
| Density | 0.984 g/cm |
| Melting point | −15 °C; 5 °F; 258 K |
| Solubility in water | Insoluble |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Flash point | 54.4 °C (129.9 °F; 327.5 K) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 2 ppm (12 mg/m) |
| REL (Recommended) | TWA 2 ppm (12 mg/m) ST 3 ppm (18 mg/m) |
| IDLH (Immediate danger) | N.D. |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Allyl propyl disulfide is an organosulfur compound with the chemical formula C3H5S2C3H7. It is a volatile pale-yellow liquid with a strong odor. It is a major component of onion oil and is used in food additives and flavors.
Allyl propyl disulfide is present in garlic and onion. When onion or garlic is sliced, the substance evaporates and causes eyes to irritate. When garlic or onion is cooked, it also evaporates, ridding them of the spicy taste, and leaving a sweet taste.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0020". National Institute for Occupational Safety and Health (NIOSH).
- Lawson, Larry D.; Wang, Zhen Yu J.; Hughes, Bronwyn G. "Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products" Planta Medica 1991, vol. 57, pp. 363-70. doi:10.1055/s-2006-960119
- CDC - NIOSH Pocket Guide to Chemical Hazards
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |