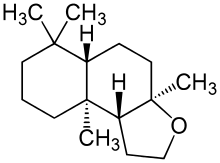
 | |
| Names | |
|---|---|
| Preferred IUPAC name (3aR,5aS,9aS,9bR)-3a,6,6,9a-Tetramethyldodecahydronaphthofuran | |
| Other names
Ambrox (Firmenich) Ambrofix (Givaudan) Ambroxan (Kao) Ambermox Orcanox (3aR-(3aα,5aβ,9aα,9bβ))-Dodecahydro-3a,6,6,9a-tetra-methylnaphtho(2,1-b)furan; Naphtho(2,1-b)furan, dodecahydro-3a,6,6,9a-tetramethyl-,; 8α, 12-Oxido-13,14,15,16-tetranorlabdane; 1,5,5,9-Tetramethyl-13-oxatricyclo(8.3.0.0(4,9))tridecane | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.147 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H28O |
| Molar mass | 236.399 g·mol |
| Density | 0.939 g/cm |
| Melting point | 75 °C (167 °F; 348 K) |
| Boiling point | 120 °C (248 °F; 393 K) (1.40 mm Hg) |
| Solubility in water | insoluble |
| Solubility in ethanol | soluble |
| Refractive index (nD) | 1.48 |
| Hazards | |
| Flash point | 161 °C (322 °F; 434 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
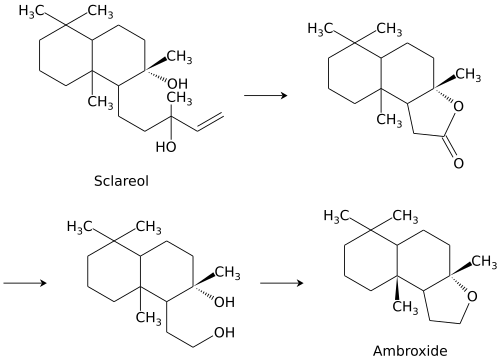
Ambroxide, widely known by the brand name Ambroxan, is a naturally occurring terpenoid and one of the key constituents responsible for the odor of ambergris. It is an autoxidation product of ambrein. Ambroxide is used in perfumery for creating ambergris notes and as a fixative. Small amounts (< 0.01 ppm) are used as a flavoring in food.
Synthesis
Ambroxide is synthesized from sclareol, a component of the essential oil of clary sage. Sclareol is oxidatively degraded to a lactone, which is hydrogenated to the corresponding diol. The resulting compound is dehydrated to form ambroxide.
References
- "Apply for a Trademark. Search a Trademark". trademarkia.com. Retrieved 25 February 2018.
- ^ Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 72
- George A. Burdock (2010), "1,5,5,9-TETRAMETHYL-13-OXATRICYCLO-(8.3.0.0(4,9)) TRIDECANE", Fenaroli's Handbook of Flavor Ingredients (6th ed.), CRC Press, p. 1895
- Brian M Lawrence (2003). Essential Oils 1995-2000. Allured Pub. ISBN 0-931710-94-4.
- Dub, Pavel A.; Gordon, John C. (2018). "The role of the metal-bound N–H functionality in Noyori-type molecular catalysts". Nature Reviews Chemistry. 2 (12): 396–408. doi:10.1038/s41570-018-0049-z. S2CID 106394152.