2, see Bromite.
 | |
 | |
| Names | |
|---|---|
| IUPAC name Bromine dioxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
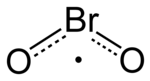
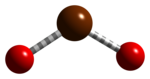
| Chemical formula | BrO2 |
| Molar mass | 111.903 g/mol |
| Appearance | unstable yellow crystals |
| Melting point | decomposes around 0°C
|
| Related compounds | |
| Other anions | Bromine monoxide Bromine trifluoride Bromine pentafluoride |
| Other cations | Oxygen difluoride Dichlorine monoxide Chlorine dioxide Iodine dioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important in the atmospheric reaction of bromine with ozone. It is similar to chlorine dioxide, the dioxide of its halogen neighbor one period higher on the periodic table.
Reactions
Bromine dioxide is formed when an electric current is passed through a mixture of bromine and oxygen gases at low temperature and pressure.
Bromine dioxide can also be formed by the treatment of bromine gas with ozone in trichlorofluoromethane at −50 °C.
When mixed with a base, bromine dioxide gives the bromide and bromate anions:
References
- ^ Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 74, ISBN 0-8493-8671-3, retrieved 17 March 2009
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, p. 447, ISBN 0-8493-0594-2
- Müller, Holger S. P.; Miller, Charles E.; Cohen, Edward A. (1997). "The rotational spectrum and molecular properties of bromine dioxide, OBrO". The Journal of Chemical Physics. 107 (20): 8292. Bibcode:1997JChPh.107.8292M. doi:10.1063/1.475030. ISSN 0021-9606.
- ^ Arora, M.G. (1997), P-Block Elements, New Delhi: Anmol Publications, p. 256, ISBN 978-81-7488-563-0, retrieved 17 March 2009
| Bromine compounds | |
|---|---|
| Br(−I) | |
| Br(−I,I) | |
| Br(I) | |
| Br(II) | |
| Br(I,V) | |
| Br(III) | |
| Br(IV) | |
| Br(V) | |
| Br(VII) | |
Categories: