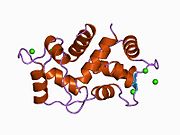
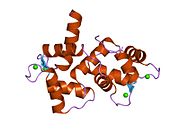
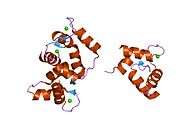
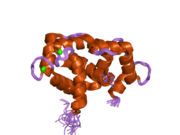
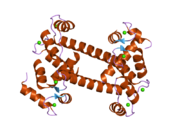
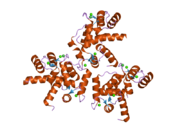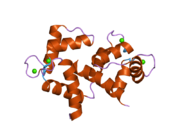Calmodulin 3 is a protein that in humans is encoded by the CALM3 gene.
CALM-3 is best known for contracting the heart muscles, and depending on whether this activity is consistent or not, other diseases could emerge as a downside. It is able to maintain or regulate in different types of biological systems, such as cytokinesis or the centrosome cycle.
Calmodulin-3 is able to perform different types of activities and roles, such as binding of calcium and significant activity in regulating an enzyme. The gene CALM-3 is likely to contribute to illnesses that may lead to death, such as Ventricular tachycardia which is associated with the ventricular tachycardia functioning in 2 directions and long QT syndrome which is associated with the QT interval in the electrocardiogram that is significantly longer than normal. In its structure, there are 2 helices that are observed in each of its helix-loop-helix and are then shaped into a perpendicular pattern due to the surface of the protein changing over time. Through transcription, the gene CALM-3 is able to perform the activity of a regulator for its own gene expression and has 6 exons, indicating that each exon has a specific function that takes place in the initiation stage. If there are potentially variants that could impact the calmodulin protein, it could affect the concentration of the Ca mediators that are a part of the protein.
Context
The CALM-3 gene, along with the protein of calmodulin, has been included in different types of experiments such as DNA isolation that is most common in laboratory animals such as rats. This gene can be detected in animals and humans, mainly through our genomes, and its specific polymorphisms can be found through different types of restriction enzymes. In hospital settings, a process named whole exome sequencing are used and are beneficial in determining whether CALM-3 is a cause of a certain disease. Because the protein calmodulin consists of 3 different genes, it may be difficult to determine exactly how the gene can cause a certain disease to occur and potentially worsen. However, there have been few mutations that were detected in the genes of the calmodulin protein such as in long QT syndrome.
Clinical significance
There is significant evidence that Calmodulin-3 may be associated with certain diseases, however there are few evidence that this particular gene contributes to diseases that can cause a sudden death as a result. In the lab experiment with rats, lambda rCB1 or hCE1 underwent DNA isolation as both of the genes included the CALM-3 gene, and was compared with 2 different genes that are more common among rats such as genes lambda SC4 and lambda SC8. As a result, although the lambda rCB1 or hCE1 gene may have different structures from the other genes that rats contain in their genomes, its coding strands were fairly similar. As the process of whole exome sequencing was used for patients with long QT syndrome, there was a certain criteria that had to be met in order to fully go through WES such as the patient having a stable or normal medical family history. Based on an electrocardiogram, the rhythms and waves can be detected and if irregular, it could lead to the pathway of long QT syndrome.
References
- ^ GRCh38: Ensembl release 89: ENSG00000160014 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "CALM3 - Calmodulin-3 - Homo sapiens (Human) - CALM3 gene & protein". www.uniprot.org. Retrieved 2022-05-18.
- ^ "CALM3 - Calmodulin-3 - Homo sapiens (Human) - CALM3 gene & protein". www.uniprot.org. Retrieved 2022-04-16.
- Zhang M, Yuan T (2011-01-24). "Molecular mechanisms of calmodulin's functional versatility". Biochemistry and Cell Biology. 76 (2–3): 313–323. doi:10.1139/o98-027. PMID 9923700.
- Koller M, Schnyder B, Strehler EE (October 1990). "Structural organization of the human CaMIII calmodulin gene". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1087 (2): 180–189. doi:10.1016/0167-4781(90)90203-E. PMID 2223880.
- Friedrich FW, Bausero P, Sun Y, Treszl A, Krämer E, Juhr D, et al. (July 2009). "A new polymorphism in human calmodulin III gene promoter is a potential modifier gene for familial hypertrophic cardiomyopathy". European Heart Journal. 30 (13): 1648–1655. doi:10.1093/eurheartj/ehp153. PMID 19429631.
- ^ SenGupta B, Friedberg F, Detera-Wadleigh SD (December 1987). "Molecular analysis of human and rat calmodulin complementary DNA clones. Evidence for additional active genes in these species". The Journal of Biological Chemistry. 262 (34): 16663–16670. doi:10.1016/S0021-9258(18)49306-4. PMID 2445749.
- ^ Reed GJ, Boczek NJ, Etheridge SP, Ackerman MJ (February 2015). "CALM3 mutation associated with long QT syndrome". Heart Rhythm. 12 (2): 419–422. doi:10.1016/j.hrthm.2014.10.035. PMC 4907373. PMID 25460178.
Further reading
- Zhang M, Yuan T (1999). "Molecular mechanisms of calmodulin's functional versatility". Biochemistry and Cell Biology. 76 (2–3): 313–323. doi:10.1139/bcb-76-2-3-313. PMID 9923700.
- Gusev NB (October 2001). "Some properties of caldesmon and calponin and the participation of these proteins in regulation of smooth muscle contraction and cytoskeleton formation". Biochemistry. Biokhimiia. 66 (10): 1112–1121. doi:10.1023/A:1012480829618. PMID 11736632. S2CID 310781.
- Benaim G, Villalobo A (August 2002). "Phosphorylation of calmodulin. Functional implications". European Journal of Biochemistry. 269 (15): 3619–3631. doi:10.1046/j.1432-1033.2002.03038.x. hdl:10261/79981. PMID 12153558.
- Chattopadhyaya R, Meador WE, Means AR, Quiocho FA (December 1992). "Calmodulin structure refined at 1.7 A resolution". Journal of Molecular Biology. 228 (4): 1177–1192. doi:10.1016/0022-2836(92)90324-D. PMID 1474585.
- Koller M, Schnyder B, Strehler EE (October 1990). "Structural organization of the human CaMIII calmodulin gene". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1087 (2): 180–189. doi:10.1016/0167-4781(90)90203-e. PMID 2223880.
- Pegues JC, Friedberg F (November 1990). "Multiple mRNAs encoding human calmodulin". Biochemical and Biophysical Research Communications. 172 (3): 1145–1149. doi:10.1016/0006-291X(90)91567-C. PMID 2244899.
- Baudier J, Mochly-Rosen D, Newton A, Lee SH, Koshland DE, Cole RD (May 1987). "Comparison of S100b protein with calmodulin: interactions with melittin and microtubule-associated tau proteins and inhibition of phosphorylation of tau proteins by protein kinase C". Biochemistry. 26 (10): 2886–2893. doi:10.1021/bi00384a033. PMID 3111527.
- Fischer R, Koller M, Flura M, Mathews S, Strehler-Page MA, Krebs J, et al. (November 1988). "Multiple divergent mRNAs code for a single human calmodulin". The Journal of Biological Chemistry. 263 (32): 17055–17062. doi:10.1016/S0021-9258(18)37497-0. PMID 3182832.
- Wawrzynczak EJ, Perham RN (August 1984). "Isolation and nucleotide sequence of a cDNA encoding human calmodulin". Biochemistry International. 9 (2): 177–185. PMID 6385987.
- Sasagawa T, Ericsson LH, Walsh KA, Schreiber WE, Fischer EH, Titani K (May 1982). "Complete amino acid sequence of human brain calmodulin". Biochemistry. 21 (10): 2565–2569. doi:10.1021/bi00539a041. PMID 7093203.
- Cook WJ, Walter LJ, Walter MR (December 1994). "Drug binding by calmodulin: crystal structure of a calmodulin-trifluoperazine complex". Biochemistry. 33 (51): 15259–15265. doi:10.1021/bi00255a006. PMID 7803388.
- Rhyner JA, Ottiger M, Wicki R, Greenwood TM, Strehler EE (October 1994). "Structure of the human CALM1 calmodulin gene and identification of two CALM1-related pseudogenes CALM1P1 and CALM1P2". European Journal of Biochemistry. 225 (1): 71–82. doi:10.1111/j.1432-1033.1994.00071.x. PMID 7925473.
- Srinivas SK, Srinivas RV, Anantharamaiah GM, Compans RW, Segrest JP (October 1993). "Cytosolic domain of the human immunodeficiency virus envelope glycoproteins binds to calmodulin and inhibits calmodulin-regulated proteins". The Journal of Biological Chemistry. 268 (30): 22895–22899. doi:10.1016/S0021-9258(18)41610-9. PMID 8226798.
- Miller MA, Mietzner TA, Cloyd MW, Robey WG, Montelaro RC (November 1993). "Identification of a calmodulin-binding and inhibitory peptide domain in the HIV-1 transmembrane glycoprotein". AIDS Research and Human Retroviruses. 9 (11): 1057–1066. doi:10.1089/aid.1993.9.1057. PMID 8312049.
- Berchtold MW, Egli R, Rhyner JA, Hameister H, Strehler EE (May 1993). "Localization of the human bona fide calmodulin genes CALM1, CALM2, and CALM3 to chromosomes 14q24-q31, 2p21.1-p21.3, and 19q13.2-q13.3". Genomics. 16 (2): 461–465. doi:10.1006/geno.1993.1211. PMID 8314583.
- Koller M, Strehler EE (April 1993). "Functional analysis of the promoters of the human CaMIII calmodulin gene and of the intronless gene coding for a calmodulin-like protein". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1163 (1): 1–9. doi:10.1016/0167-4838(93)90271-R. PMID 8476923.
- Radding W, Pan ZQ, Hunter E, Johnston P, Williams JP, McDonald JM (January 1996). "Expression of HIV-1 envelope glycoprotein alters cellular calmodulin". Biochemical and Biophysical Research Communications. 218 (1): 192–197. doi:10.1006/bbrc.1996.0034. PMID 8573130.
- Pan Z, Radding W, Zhou T, Hunter E, Mountz J, McDonald JM (September 1996). "Role of calmodulin in HIV-potentiated Fas-mediated apoptosis". The American Journal of Pathology. 149 (3): 903–910. PMC 1865159. PMID 8780394.
- Sasaki M, Uchiyama J, Ishikawa H, Matsushita S, Kimura G, Nomoto K, Koga Y (October 1996). "Induction of apoptosis by calmodulin-dependent intracellular Ca2+ elevation in CD4+ cells expressing gp 160 of HIV". Virology. 224 (1): 18–24. doi:10.1006/viro.1996.0502. PMID 8862395.
External links
- Human CALM3 genome location and CALM3 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P0DP23 (Calmodulin-1) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: P0DP24 (Calmodulin-2) at the PDBe-KB.
| PDB gallery | |
|---|---|
|