| This article's factual accuracy is disputed. Relevant discussion may be found on the talk page. Please help to ensure that disputed statements are reliably sourced. (August 2021) (Learn how and when to remove this message) |
 | |
| Names | |
|---|---|
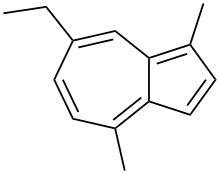
| Preferred IUPAC name 7-Ethyl-1,4-dimethylazulene | |
| Other names 1,4-Dimethyl-7-ethylazulene; Ba 2784; Camazulene; Chamazulen; Dimethulen; Dimethulene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.682 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H16 |
| Molar mass | 184.282 g·mol |
| Appearance | Blue oil |
| Density | 0.9883 (at 20 °C) |
| Boiling point | 161 °C (322 °F; 434 K) (at 12 mmHg) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 3 g/kg (i.m., mouse) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Chamazulene is an aromatic chemical compound with the molecular formula C14H16 found in a variety of plants including in chamomile (Matricaria chamomilla), wormwood (Artemisia absinthium), and yarrow (Achillea millefolium). It is a blue-violet derivative of azulene which is biosynthesized from the sesquiterpene matricin.

Biosynthesis of chamazulene (3) from matricin (1) via a carboxylic acid of chamazulene (2).
Chamazulene has anti-inflammatory properties in vivo and inhibits the CYP1A2 enzyme.
References
- ^ The Merck Index, 11th Edition, 2031
- ^ Safayhi, H; Sabieraj, J; Sailer, ER; Ammon, HP (1994). "Chamazulene: An antioxidant-type inhibitor of leukotriene B4 formation". Planta Medica. 60 (5): 410–3. doi:10.1055/s-2006-959520. PMID 7997466.
This article is a stub. You can help Misplaced Pages by expanding it. |