 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Copper(II) perchlorate | |
| Other names Cupric perchlorate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.978 |
| EC Number |
|
| PubChem CID |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
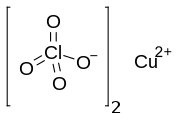
| Chemical formula | Cu(ClO4)2 |
| Molar mass | 262.447 g/mol (anhydrous) 370.539 g/mol (hexahydrate) |
| Appearance | Blue crystalline hygroscopic solid (hexahydrate) |
| Odor | odorless |
| Density | 2.225 g/cm (hexahydrate) |
| Melting point | 82 °C (180 °F; 355 K) (hexahydrate) |
| Boiling point | 120 °C (248 °F; 393 K) (hexahydrate) |
| Solubility in water | 146 g/(100 ml) (30°C) |
| Refractive index (nD) | 1.505 (hexahydrate) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H272, H315, H319, H335 |
| Precautionary statements | P210, P220, P221, P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P405, P501 |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 1 mg/m (as Cu) |
| REL (Recommended) | TWA 1 mg/m (as Cu) |
| IDLH (Immediate danger) | TWA 100 mg/m (as Cu) |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Copper(II) perchlorate is an inorganic compound with the chemical formula Cu(ClO4)2(H2O)x. The anhydrous solid is rarely encountered but several hydrates are known. Most important is the perchlorate salt of the aquo complex copper(II) perchlorate hexahydrate, [Cu(H2O)6].
Infrared spectroscopic studies of anhydrous copper(II) perchlorate provided some of the first evidence for the binding of perchlorate anion to a metal ion. The structure of this compound was eventually deduced by X-ray crystallography. Copper resides in a distorted octahedral environment and the perchlorate ligands bridge between the Cu(II) centers.
Safety
Like other perchlorates, copper(II) perchlorate is a strong oxidant.
References
- "Copper(Ii) Perchlorate Hexahydrate | 10294-46-9".
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- Gallucci, J. C.; Gerkin, R. E. (1989). "Structure of copper(II) perchlorate hexahydrate". Acta Crystallogr. C. 45 (9): 1279–1284. doi:10.1107/S0108270189000818. PMID 2557867.
- Hathaway, B. J.; Underhill, A. E. (1961). "592. The infrared spectra of some transition-metal perchlorates". Journal of the Chemical Society (Resumed): 3091. doi:10.1039/JR9610003091.
- Pascal, Jean-Louis; Favier, Frédéric (1998). "Inorganic Perchlorato Complexes". Coordination Chemistry Reviews. 178–180: 865–902. doi:10.1016/S0010-8545(98)00102-7.
| Copper compounds | |
|---|---|
| Cu(0,I) | |
| Cu(I) | |
| Cu(I,II) | |
| Cu(II) | |
| Cu(III) | |
| Cu(IV) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |