 | |
| Clinical data | |
|---|---|
| Trade names | Zefnart |
| Other names | M-732; piritetrate |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
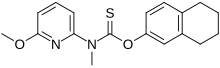
| Formula | C18H20N2O2S |
| Molar mass | 328.43 g·mol |
Liranaftate (trade name Zefnart) is a topical antifungal drug. It is used as a 2% cream used to treat tinea pedis (athlete's foot), tinea corporis (ringworm), and tinea cruris (jock itch). It was approved for use in Japan in August 2000.
Liranaftate works by inhibiting the fungal enzyme squalene epoxidase that is necessary for the fungus to synthesize sterols which are essential for cell membrane integrity.
References
- Koga H, Nanjoh Y, Makimura K, Tsuboi R (2009). "In vitro antifungal activities of luliconazole, a new topical imidazole". Medical Mycology. 47 (6): 640–7. doi:10.1080/13693780802541518. PMID 19115136.
- "Torii Pharmaceutical to Launch Antifungal Agent for External Use, "ZEFNART SOLUTION 2%", in Japan" (Press release). Torii Pharmaceutical Co. Retrieved June 27, 2021.
- "Liranaftate". ncats.io. Retrieved June 27, 2021.
- "Liranaftate". Adis Insight. Retrieved June 27, 2021.
- CID 3936 from PubChem
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |