| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Myricanone" – news · newspapers · books · scholar · JSTOR (August 2024) |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H24O5 |
| Molar mass | 356.41 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
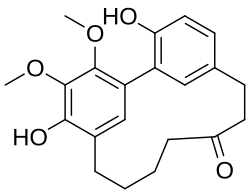
Myricanone is a cyclic diarylheptanoid isolated from the bark of Myrica rubra (Myricaceae).
References
- Akazawa, H; Fujita, Y; Banno, N; Watanabe, K; Kimura, Y; Manosroi, A; Manosroi, J; Akihisa, T (2010). "Three new cyclic diarylheptanoids and other phenolic compounds from the bark of Myrica rubra and their melanogenesis inhibitory and radical scavenging activities". Journal of Oleo Science. 59 (4): 213–221. doi:10.5650/jos.59.213. PMID 20299768.
| Types of natural diarylheptanoids | |
|---|---|
| Linear | |
| Cyclic | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |