| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-Pyrazole | |||
| Systematic IUPAC name 1,2-Diazacyclopenta-2,4-diene | |||
| Other names 1,2-Diazole | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 103775 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.471 | ||
| EC Number |
| ||
| Gmelin Reference | 1360 | ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C3H4N2 | ||
| Molar mass | 68.079 g·mol | ||
| Melting point | 66 to 70 °C (151 to 158 °F; 339 to 343 K) | ||
| Boiling point | 186 to 188 °C (367 to 370 °F; 459 to 461 K) | ||
| Basicity (pKb) | 11.5 | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Danger | ||
| Hazard statements | H302, H311, H315, H318, H319, H335, H372, H412 | ||
| Precautionary statements | P260, P261, P262, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P319, P321, P330, P332+P317, P337+P317, P361+P364, P362+P364, P403+P233, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
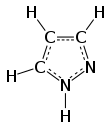
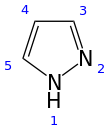
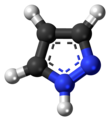
Pyrazole is an organic compound with the formula (CH)3N2H. It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest.
Properties
Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å
Substituted pyrazoles
Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
Preparation and reactions
Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation:
Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine (Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole:
- CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O
A wide variety of pyrazoles can be made so:
History
The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane.
Conversion to scorpionates
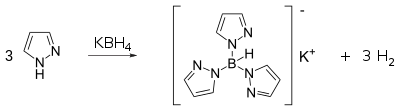
Pyrazoles react with potassium borohydride to form a class of ligands known as scorpionate. Pyrazole itself reacts with potassium borohydride at high temperatures (~200 °C) to form a tridentate ligand known as Tp ligand:
3,5-Diphenyl-1H-pyrazole
3,5-Diphenyl-1H-pyrazole is produced when (E)-1,3-diphenylprop-2-en-1-one is reacted with hydrazine hydrate in the presence of elemental sulfur or sodium persulfate, or by using a hydrazone in which case an azine is produced as a by-product.
Occurrence and uses

In 1959, the first natural pyrazole, 1-pyrazolyl-alanine, was isolated from seeds of watermelons.
In medicine, derivatives of pyrazole are widely used, including celecoxib and similar COX-2 inhibitors, zaleplon, betazole, and CDPPB. The pyrazole ring is found within a variety of pesticides as fungicides, insecticides and herbicides, including fenpyroximate, fipronil, tebufenpyrad and tolfenpyrad. Pyrazole moieties are listed among the highly used ring systems for small molecule drugs by the US FDA
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is used in the manufacture of six commercial fungicides which are inhibitors of succinate dehydrogenase.
Pyrazole is an inhibitor of the alcohol dehydrogenase enzyme, and, as such, is used as an adjuvant with ethanol, to induce alcohol dependency in experimental laboratory mice.
See also
- 3,5-dimethylpyrazole
- Pyrazolidine, fully saturated analogue
- imidazole, structural analogue of pyrazole with two non-adjacent nitrogen atoms.
- isoxazole, another analogue, the nitrogen atom in position 1 replaced by oxygen.
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 141. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- "Pyrazole". pubchem.ncbi.nlm.nih.gov. Retrieved 17 February 2024.
- "Dissociation constants of organic acids and bases" (PDF). Archived (PDF) from the original on 12 July 2017.
- La Cour, Troels; Rasmussen, Svend Erik; Hopf, Henning; Waisvisz, Jacques M.; Van Der Hoeven, Marcel G.; Swahn, Carl-Gunnar (1973). "The Structure of Pyrazole, C3H4N2, at 295 K and 108 K as determined by X-Ray Diffraction". Acta Chemica Scandinavica. 27: 1845–1854. doi:10.3891/acta.chem.scand.27-1845.
- Eicher, T.; Hauptmann, S. (2003). The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications (2nd ed.). Wiley-VCH. ISBN 3-527-30720-6.
- Schmidt, Andreas; Dreger, Andrij (2011). "Recent Advances in the Chemistry of Pyrazoles. Properties, Biological Activities, and Syntheses". Curr. Org. Chem. 15 (9): 1423–1463. doi:10.2174/138527211795378263.
- ^ Nozari, Mohammad; Addison, Anthony W.; Reeves, Gordan T.; Zeller, Matthias; Jasinski, Jerry P.; Kaur, Manpreet; Gilbert, Jayakumar G.; Hamilton, Clifton R.; Popovitch, Jonathan M.; Wolf, Lawrence M.; Crist, Lindsay E.; Bastida, Natalia (2018). "New Pyrazole- and Benzimidazole-derived Ligand Systems". Journal of Heterocyclic Chemistry. 55 (6): 1291–1307. doi:10.1002/jhet.3155.
- Johnson, William S.; Highet, Robert J. (1951). "3,5-Dimethylpyrazole". Organic Syntheses. 31: 43. doi:10.15227/orgsyn.031.0043.
- Knorr, L. (1883). "Action of ethyl acetoacetate on phenylhydrazine. I". Chemische Berichte. 16: 2597–2599. doi:10.1002/cber.188301602194.
- von Pechmann, Hans (1898). "Pyrazol aus Acetylen und Diazomethan". Berichte der deutschen chemischen Gesellschaft (in German). 31 (3): 2950–2951. doi:10.1002/cber.18980310363.
- Outirite, Moha; Lebrini, Mounim; Lagrenée, Michel; Bentiss, Fouad (2008). "New one step synthesis of 3,5-disubstituted pyrazoles under microwave irradiation and classical heating". Journal of Heterocyclic Chemistry. 45 (2): 503–505. doi:10.1002/jhet.5570450231.
- Zhang, Ze; Tan, Ya-Jun; Wang, Chun-Shan; Wu, Hao-Hao (2014). "One-pot synthesis of 3,5-diphenyl-1H-pyrazoles from chalcones and hydrazine under mechanochemical ball milling". Heterocycles. 89 (1): 103–112. doi:10.3987/COM-13-12867.
- Lasri, Jamal; Ismail, Ali I. (2018). "Metal-free and FeCl3-catalyzed synthesis of azines and 3,5-diphenyl-1H-pyrazole from hydrazones and/or ketones monitored by high resolution ESI-MS". Indian Journal of Chemistry, Section B. 57B (3): 362–373.
- Fowden; Noe; Ridd; White (1959). Proc. Chem. Soc.: 131.
{{cite journal}}: Missing or empty|title=(help) - Noe, F. F.; Fowden, L.; Richmond, P. T. (1959). "alpha-Amino-beta-(pyrazolyl-N) propionic acid: a new amino-acid from Citrullus vulgaris (water melon)". Nature. 184 (4688): 69–70. Bibcode:1959Natur.184...69B. doi:10.1038/184069a0. PMID 13804343. S2CID 37499048.
- ^ Kabi, Arup K.; Sravani, Sattu; Gujjarappa, Raghuram; et al. (2022). "Overview on Biological Activities of Pyrazole Derivatives". Nanostructured Biomaterials. Materials Horizons: From Nature to Nanomaterials. pp. 229–306. doi:10.1007/978-981-16-8399-2_7. ISBN 978-981-16-8398-5.
- Faria, Jéssica Venância; Vegi, Percilene Fazolin; Miguita, Ana Gabriella Carvalho; dos Santos, Maurício Silva; Boechat, Nubia; Bernardino, Alice Maria Rolim (1 November 2017). "Recently reported biological activities of pyrazole compounds". Bioorganic & Medicinal Chemistry. 25 (21): 5891–5903. doi:10.1016/j.bmc.2017.09.035. ISSN 0968-0896. PMID 28988624.
- FAO
- Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J Med Chem 2014, 57, 5845.
- Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". In Lamberth, Clemens; Dinges, Jürgen (eds.). Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- Jeschke, Peter (2021). "Current Trends in the Design of Fluorine-Containing Agrochemicals". In Szabó, Kálmán; Selander, Nicklas (eds.). Organofluorine Chemistry. Wiley. pp. 363–395. doi:10.1002/9783527825158.ch11. ISBN 9783527347117. S2CID 234149806.
- Xiao, T.; Chen, Y.; Boisvert, A.; Cole, M.; Kimbrough, A. (2023). "Chronic Intermittent Ethanol Vapor Exposure Paired with Two-Bottle Choice to Model Alcohol Use Disorder". Journal of Visualized Experiments (196). doi:10.3791/65320. PMC 11164185. PMID 37427930.
Further reading
A. Schmidt; A. Dreger (2011). "Recent Advances in the Chemistry of Pyrazoles. Part 2. Reactions and N-Heterocyclic Carbenes of Pyrazole". Curr. Org. Chem. 15 (16): 2897–2970. doi:10.2174/138527211796378497.
Categories: