 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.039.223 |
| Chemical and physical data | |
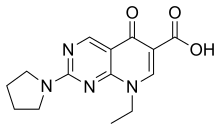
| Formula | C14H16N4O3 |
| Molar mass | 288.307 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Piromidic acid is a quinolone antibiotic.
References
- Minami S, Shono T, Matsumoto JI (1971). "Pyrido pyrimidine Antibacterial Agents. II. Piromidic Acid and Related Compounds". Chemical and Pharmaceutical Bulletin. 19 (7): 1426–1432. doi:10.1248/cpb.19.1426.
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |