 | |
| Names | |
|---|---|
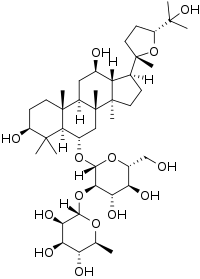
| IUPAC name (24R)-3β,12β,25-Trihydroxy-20,24-epoxydammaran-6α-yl α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | |
| Systematic IUPAC name (2S,3R,4R,5R,6S)-2-{-3a,3b,6,6,9a-pentamethylhexadecahydro-5H-cyclopentaphenanthren-5-yl}oxy)-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy}-6-methyloxane-3,4,5-triol | |
| Other names Ginsenoside A1 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.208.747 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C42H72O14 |
| Molar mass | 801.024 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Pseudoginsenoside F11 is a chemical natural product found in American ginseng (Panax quinquefolius) but not in Asian ginseng (Panax ginseng), although it has similar properties to the Asian ginseng compound ginsenoside Rf. The molecule is a triterpenoid saponin member of the dammarane family and contains a four-ring rigid skeleton. Compounds in the ginsenoside family are found almost exclusively in plants of the genus Panax. A wide variety of difficult-to-characterize in vitro effects have been reported for the compounds in isolation. Pseudoginsenoside F11 and its derivatives are sometimes referred to as having an ocotillol-type skeleton structure.
Studies in mice have identified antagonistic effects on the actions of other well-characterized drugs, such as scopolamine, morphine, and methamphetamine.
References
- ^ Qi, LW; Wang, CZ; Yuan, CS (June 2011). "Ginsenosides from American ginseng: chemical and pharmacological diversity". Phytochemistry. 72 (8): 689–99. doi:10.1016/j.phytochem.2011.02.012. PMC 3103855. PMID 21396670.
- Attele, AS; Wu, JA; Yuan, CS (1 December 1999). "Ginseng pharmacology: multiple constituents and multiple actions". Biochemical Pharmacology. 58 (11): 1685–93. doi:10.1016/s0006-2952(99)00212-9. PMID 10571242.
- Christensen, LP (2009). "Ginsenosides chemistry, biosynthesis, analysis, and potential health effects". Advances in Food and Nutrition Research. 55: 1–99. doi:10.1016/S1043-4526(08)00401-4. PMID 18772102.
- Fuzzati, N (5 December 2004). "Analysis methods of ginsenosides". Journal of Chromatography B. 812 (1–2): 119–33. doi:10.1016/j.jchromb.2004.07.039. PMID 15556492.
- Li, Z; Guo, YY; Wu, CF; Li, X; Wang, JH (April 1999). "Protective effects of pseudoginsenoside-F11 on scopolamine-induced memory impairment in mice and rats". The Journal of Pharmacy and Pharmacology. 51 (4): 435–40. doi:10.1211/0022357991772484. PMID 10385216. S2CID 12905089.
- Li, Z; Wu, CF; Pei, G; Guo, YY; Li, X (July 2000). "Antagonistic effect of pseudoginsenoside-F11 on the behavioral actions of morphine in mice". Pharmacology Biochemistry and Behavior. 66 (3): 595–601. doi:10.1016/s0091-3057(00)00260-4. PMID 10899376. S2CID 40882518.
- Hao, Y; Yang, JY; Wu, CF; Wu, MF (April 2007). "Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial prefrontal cortex in mice". Pharmacology Biochemistry and Behavior. 86 (4): 660–6. doi:10.1016/j.pbb.2007.02.011. PMID 17368734. S2CID 32919832.
- Wu, CF; Liu, YL; Song, M; Liu, W; Wang, JH; Li, X; Yang, JY (August 2003). "Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice". Pharmacology Biochemistry and Behavior. 76 (1): 103–9. doi:10.1016/s0091-3057(03)00215-6. PMID 13679222. S2CID 20865626.