| |||
| Names | |||
|---|---|---|---|
| IUPAC name Selenium hexafluoride | |||
| Other names Selenium(VI) fluoride, Selenium fluoride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.149.506 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | SeF6 | ||
| Molar mass | 192.9534 g/mol | ||
| Appearance | colourless gas | ||
| Density | 7.887 g/L | ||
| Melting point | −39 °C (−38 °F; 234 K) | ||
| Boiling point | −34.5 °C (−30.1 °F; 238.7 K) sublimes | ||
| Solubility in water | insoluble | ||
| Vapor pressure | >1 atm (20°C) | ||
| Magnetic susceptibility (χ) | −51.0·10 cm/mol | ||
| Refractive index (nD) | 1.895 | ||
| Structure | |||
| Crystal structure | Orthorhombic, oP28 | ||
| Space group | Pnma, No. 62 | ||
| Coordination geometry | octahedral (Oh) | ||
| Dipole moment | 0 | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
-1030 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | toxic, corrosive | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LCLo (lowest published) | 10 ppm (rat, 1 hr) 10 ppm (mouse, 1 hr) 10 ppm (guinea pig, 1 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 0.05 ppm (0.4 mg/m) | ||
| REL (Recommended) | TWA 0.05 ppm | ||
| IDLH (Immediate danger) | 2 ppm | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.
Structure, preparation, and reactions
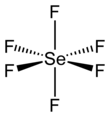
SeF6 has octahedral molecular geometry with an Se−F bond length of 168.8 pm. In terms of bonding, it is hypervalent.
SeF6 can be prepared from the elements. It also forms by the reaction of bromine trifluoride (BrF3) with selenium dioxide. The crude product can be purified by sublimation.
The relative reactivity of the hexafluorides of S, Se, and Te follows the order TeF6 > SeF6 > SF6, the latter being completely inert toward hydrolysis until high temperatures. SeF6 also resists hydrolysis. The gas can be passed through 10% NaOH or KOH without change, but reacts with gaseous ammonia at 200 °C.
Safety
Although selenium hexafluoride is quite inert and slow to hydrolyze, it is toxic even at low concentrations, especially by longer exposure. In the U.S., OSHA and ACGIH standards for selenium hexafluoride exposure is an upper limit of 0.05 ppm in air averaged over an eight-hour work shift. Additionally, selenium hexafluoride is designated as IDLH chemical with a maximum allowed exposure limit of 2 ppm.
References
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0551". National Institute for Occupational Safety and Health (NIOSH).
- ^ Wiberg, E.; Holleman, A. F. (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5.
- "Selenium hexafluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Material Safety" (PDF). Retrieved 2010-07-24.
- Langner, B. E. "Selenium and Selenium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_525. ISBN 978-3-527-30673-2.
- Yost, D. M.; Simons, J. H. (1939). "Sulfur, Selenium, and Tellurium Hexafluorides". Inorganic Syntheses. Vol. 1. pp. 121–122. doi:10.1002/9780470132326.ch44. ISBN 9780470132326.
- Krebs, B.; Bonmann, S.; Eidenschink, I. (1994). "Selenium-Inorganic Chemistry". In King, R. B. (ed.). Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 0-471-93620-0.
- "Medical Management Guidelines for Selenium Hexafluoride (SeF6)". CDC ATSDR. Archived from the original on May 28, 2010. Retrieved 2010-07-24.
- Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
External links
- ATSDR ToxFAQs - Selenium Hexafluoride U.S. Department of Health and Human Services
- CDC - NIOSH Pocket Guide to Chemical Hazards U.S. Department of Health and Human Services
- WebBook page for SeF6
| Binary hexafluorides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Known binary hexafluorides |
| ||||||||
| Predicted binary hexafluorides |
| ||||||||
| Selenium compounds | |
|---|---|
| Se(−II) | |
| Se(0,I) | |
| Se(I) | |
| Se(II) | |
| Se(IV) | |
| Se(VI) | |
| Se(IV,VI) | |