 | |
| Names | |
|---|---|
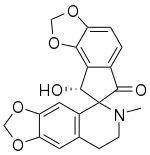
| IUPAC name 8'-Hydroxy-6-methylspirodioxoloisoquinoline-5,7'-8H-cyclopentabenzodioxole]-6'-one | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H17NO6 |
| Molar mass | 367.357 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Sibiricine is a bioactive isoquinoline alkaloid isolated from Corydalis crispa (Fumariaceae), which is a Bhutanese medicinal plant from the Himalayas.
Using high resolution mass spectrometry, the molecular formula of sibiricine is determined to be C20H17NO6. The IUPAC name for sibiricine is 8'-hydroxy-6-methylspirodioxoloisoquinoline-5,7'-8H-cyclopentabenzodioxole]-6'-one. The proton nuclear magnetic resonance (PMR) spectrum of sibiricine at 100 MHz shows that sibiricine is structurally related to ochrobirine and ochotensine. With the exception of sibiricine, 8 other alkaloids are extracted by investigating Corydalis crispa. These isoquinoline alkaloids are protopine, 13-oxoprotopine, 13-oxocryptopine, stylopine, coreximine, rheagenine, ochrobirine, and bicuculline.
References
- Southon, Ian W.; Buckingham, John (15 January 1989). Dictionary of Alkaloids, Second Edition with CD-ROM. CRC Press. p. 971. ISBN 978-0-412-24910-5.
- "KNApSAcK Metabolite Information - C00029012". www.knapsackfamily.com.
- ^ Wangchuk, P.; Keller, P. A.; Pyne, S. G.; Sastraruji, T.; Taweechotipatr, M.; Rattanajak, R.; Tonsomboon, A.; Kamchonwongpaisan, S. (2012). "Phytochemical and biological activity studies of the Bhutanese medicinal plant Corydalis crispa". Natural Product Communications. 7 (5): 575–80. doi:10.1177/1934578X1200700507. PMID 22799079.
- Wangchuk, Phurpa; Giacomin, Paul R.; Pearson, Mark S.; Smout, Michael J.; Loukas, Alex (2016). "Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms". Scientific Reports. 6: 32101. Bibcode:2016NatSR...632101W. doi:10.1038/srep32101. PMC 5004179. PMID 27572696.
- ^ Manske, R. H. F.; Rodrigo, R.; MacLean, D. B.; Gracey, D. E. F.; Saunders, J. K. (1969). "Structure of sibiricine, an alkaloid of Corydalissibirica". Canadian Journal of Chemistry. 47 (19): 3585–3588. doi:10.1139/v69-592.
- CID 632652 from PubChem