 | |
| Names | |
|---|---|
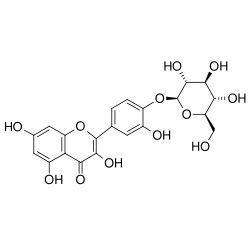
| IUPAC name 4′-(β-D-Glucopyranosyloxy)-3,3′,5,7-tetrahydroxyflavone | |
| Systematic IUPAC name 3,5,7-Trihydroxy-2-(3-hydroxy-4-{oxy}phenyl)-4H-1-benzopyran-4-one | |
| Other names
Spiraeosid Spiraein Quercetin-4'-glucoside Quercetin 4'-O-glucoside Quercetin-4-O-β-D-glucoside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 68011 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.039.634 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H20O12 |
| Molar mass | 464.37 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Spiraeoside is a chemical compound. It can be isolated from flowers of Filipendula ulmaria (L.) (a.k.a. Spiraea ulmaria or meadowsweet) or from the garden onion (Allium cepa).
Spiraeoside is the 4'-O-glucoside of quercetin.
References
- Williamson, Gary; Plumb, Geoff W.; Uda, Yasushi; Price, Keith R.; Rhodes, Michael J.C. (1996). "Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells". Carcinogenesis. 17 (11): 2385–7. doi:10.1093/carcin/17.11.2385. PMID 8968052.
- Olsson, Marie E.; Gustavsson, Karl-Erik; Vågen, Ingunn M. (2010). "Quercetin and Isorhamnetin in Sweet and Red Cultivars of Onion (Allium cepaL.) at Harvest, after Field Curing, Heat Treatment, and Storage". Journal of Agricultural and Food Chemistry. 58 (4): 2323–30. doi:10.1021/jf9027014. PMID 20099844.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |