 | |
| Names | |
|---|---|
| Other names tellurium(IV) iodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.282 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | TeI4 |
| Molar mass | 635.218 g/mol |
| Appearance | black crystals |
| Density | 5.05 g/cm, solid |
| Melting point | 280 °C (536 °F; 553 K) |
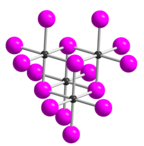
| Structure | |
| Crystal structure | orthorhombic |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H312, H314, H332 |
| Precautionary statements | P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P322, P330, P363, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
Preparation
Tellurium tetraiodide can be prepared by reacting Te and iodomethane, CH3I. In the vapour TeI4 dissociates:
- TeI4 → TeI2 + I2
It can be also obtained by reacting telluric acid with hydrogen iodide.
- Te(OH)6 + HI → TeI4 + I2 + 6 H2O
It can also be obtained by reacting the elements, which can also produce tellurium diiodide and tellurium monoiodide, depending on the reaction conditions:
- Te + 2 I2 → TeI4
- TeI4 → TeI2 + I2
Properties
Tellurium tetraiodide is an iron-gray solid that decomposes slowly in cold water and quickly in warm water to form tellurium dioxide and hydrogen iodide. It is stable even in moist air and decomposes when heated, releasing iodine. It is soluble in hydriodic acid to form H and it is slightly soluble in acetone.
Tellurium tetraiodide is a conductor when molten, dissociating into the ions TeI3 and I. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
- TeI4 + 2 CH3CN → (CH3CN)2TeI3 + I
Five modifications of tellurium tetraiodide are known, all of which are composed of tetrameric molecules. The δ form is the most thermodynamically stable form. This is structurally derived (as well as the α, β and γ forms) from the ε form.
References
- "Tellurium tetraiodide". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- ^ Handbuch der präparativen anorganischen Chemie. 1 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1975. p. 435. ISBN 978-3-432-02328-1.
- Hagen, A. P. (2009-09-17). Inorganic Reactions and Methods, The Formation of Bonds to Halogens (Part 1). John Wiley & Sons. ISBN 978-0-470-14538-8.
- Tellurium(IV) iodide, 99% (metals basis) at AlfaAesar, accessed on 2013-12-17 (PDF) (JavaScript required).
- Riedel, Erwin; Janiak, Christoph (2011). Anorganische Chemie: Zusatzmaterial online. Studium (8. Aufl ed.). Berlin: de Gruyter. ISBN 978-3-11-022567-9.
| Tellurium compounds | |
|---|---|
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||