 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.045.006 |
| Chemical and physical data | |
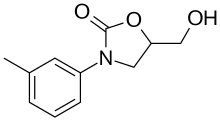
| Formula | C11H13NO3 |
| Molar mass | 207.229 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Toloxatone (Humoryl) is an antidepressant launched in 1984 in France by Sanofi Aventis for the treatment of depression. It was discontinued in 2002. It acts as a selective reversible inhibitor of MAO-A (RIMA).
Synthesis
The reaction between glycidol (1) and m-toluidine (2) gives 3-m-toluidinopropane-1,2-diol (3). Treatment with diethyl carbonate (4) in the presence of sodium methoxide leads to an intermolecular cycloaddition to give tomoxatone.
See also
References
- "Humoryl 200mg gelule 30".
- Berlin I, Zimmer R, Thiede HM, et al. (December 1990). "Comparison of the monoamine oxidase inhibiting properties of two reversible and selective monoamine oxidase-A inhibitors moclobemide and toloxatone, and assessment of their effect on psychometric performance in healthy subjects". British Journal of Clinical Pharmacology. 30 (6): 805–16. doi:10.1111/j.1365-2125.1990.tb05445.x. PMC 1368300. PMID 1705137.
- Sungurbey, K .; Castaer, J .; Toloxatone. Drugs Fut 1976, 1, 12, 569.
- Douzon, C.; Fauvan, C.; Une nouvelle serie d'antidpresseurs: les derivs de l'hydroxymthyl-5-oxazolidinone-2. Chimie Thrapeutique 1973, 3, 324-327.
- DE2012120 Claude P Fauran, Guy M Raynaud, Rene A Oliver, Colette A Douzon, U.S. patent 3,655,687 (1972 to Delalande Sa).
| Anxiolytics (N05B) | |
|---|---|
| 5-HT1ARTooltip 5-HT1A receptor agonists | |
| GABAARTooltip GABAA receptor PAMsTooltip positive allosteric modulators |
|
| Hypnotics | |
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Antipsychotics | |
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| Monoamine metabolism modulators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-specific |
| ||||||||||
| Phenethylamines (dopamine, epinephrine, norepinephrine) |
| ||||||||||
| Tryptamines (serotonin, melatonin) |
| ||||||||||
| Histamine |
| ||||||||||
| See also: Receptor/signaling modulators • Adrenergics • Dopaminergics • Melatonergics • Serotonergics • Monoamine reuptake inhibitors • Monoamine releasing agents • Monoamine neurotoxins | |||||||||||
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |
