 | |
| Clinical data | |
|---|---|
| Other names | Trestolone 17β-enanthate; MENT enanthate; 7α-Methyl-19-nortestosterone 17β-enanthate; 7α-Methylestr-4-en-17β-ol-3-one 17β-enanthate |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H40O3 |
| Molar mass | 400.603 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
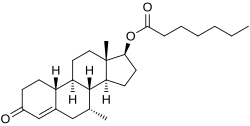
Trestolone enanthate, also known as 7α-methyl-19-nortestosterone 17β-enanthate (MENT enanthate), is an androgen and anabolic steroid (AAS) and progestogen which was never marketed. It is an androgen ester; specifically, it is the C17β enanthate (heptanoate) ester of trestolone (7α-methylestr-4-en-17β-ol-3-one). Trestolone enanthate has low affinity for sex hormone-binding globulin (SHBG), similarly to testosterone enanthate.
See also
References
- ^ Cunningham GR, Tindall DJ, Lobl TJ, Campbell JA, Means AR (September 1981). "Steroid structural requirements for high affinity binding to human sex steroid binding protein (SBP)". Steroids. 38 (3): 243–62. doi:10.1016/0039-128X(81)90061-1. PMID 7197818. S2CID 2702353.
- ^ Bursi R, Grootenhuis A, van der Louw J, Verhagen J, de Gooyer M, Jacobs P, Leysen D (March 2003). "Structure-activity relationship study of human liver microsomes-catalyzed hydrolysis rate of ester prodrugs of MENT by comparative molecular field analysis (CoMFA)". Steroids. 68 (3): 213–220. doi:10.1016/S0039-128X(02)00186-1. PMID 12628684. S2CID 32309966.
| Estrogen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| ERTooltip Estrogen receptor |
| ||||||
| GPERTooltip G protein-coupled estrogen receptor |
| ||||||
| Progesterone receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| PRTooltip Progesterone receptor |
| ||||||
| mPRTooltip Membrane progesterone receptor (PAQRTooltip Progestin and adipoQ receptor) |
| ||||||
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |