 | |
| Names | |
|---|---|
| Other names NaBH(OAc)3; STAB; STABH; Sodium triacetoxyhydroborate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.115.747 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
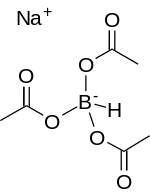
| Chemical formula | Na[(CH3COO)3BH] |
| Molar mass | 211.94 g·mol |
| Appearance | White powder |
| Density | 1.20 g/cm |
| Melting point | 116 to 120 °C (241 to 248 °F; 389 to 393 K) decomposes |
| Solubility in water | decomposition |
| Structure | |
| Coordination geometry | 4 at boron atom |
| Molecular shape | Tetrahedral at boron atom |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
| Other anions | Sodium cyanoborohydride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na[(CH3COO)3BH]. Like other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid:
- Na[BH4] + 3 CH3COOH → Na[(CH3COO)3BH] + 3 H2
Comparison with related reagents
Sodium triacetoxyborohydride is a milder reducing agent than sodium borohydride or even sodium cyanoborohydride. It reduces aldehydes but not most ketones. It is especially suitable for reductive aminations of aldehydes and ketones.
However, unlike sodium cyanoborohydride, the triacetoxyborohydride hydrolyzes readily, nor is it compatible with methanol. It reacts only slowly with ethanol and isopropanol and can be used with these.
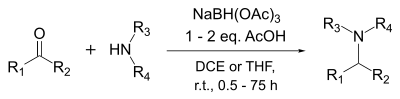
NaBH(OAc)3 may also be used for reductive alkylation of secondary amines with aldehyde-bisulfite adducts.
Monoacetoxyborohydride
The combination of Na[BH4] with carboxylic acids results in the formation of acyloxyborohydride species other than sodium triacetoxyborohydride. These modified species can perform a variety of reductions not normally associated with borohydride chemistry, such as alcohols to hydrocarbons and nitriles to primary amines.
See also
- Sodium cyanoborohydride - a slightly stronger reductant, but amenable to protic solvents
- Sodium borohydride - a stronger, cheaper reductant
- Tetramethylammonium triacetoxyborohydride
References
- Gordon W. Gribble, Ahmed F. Abdel-Magid, "Sodium Triacetoxyborohydride" Encyclopedia of Reagents for Organic Synthesis, 2007, John Wiley & Sons.doi:10.1002/047084289X.rs112.pub2
- Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, R. D. (1996). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures1". The Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/jo960057x. PMID 11667239.
- ^ Abdel-Magid, A. F.; Mehrman, S. J. (2006). "A Review on the Use of Sodium Triacetoxyborohydride in the Reductive Amination of Ketones and Aldehydes". Organic Process Research & Development. 10 (5): 971. doi:10.1021/op0601013.
- Magano, Javier; Kiser, E. Jason; Shine, Russell J.; Chen, Michael H. (2013). "Oxindole Synthesis via Palladium-catalyzed C-H Functionalization". Organic Syntheses. 90: 74. doi:10.15227/orgsyn.090.0074.
- Pandit, C. R.; Mani, N. S. (2009). "Expedient reductive amination of aldehyde bisulfite adducts". Synthesis (23): 4032–4036.
- Gribble, Gordon, W. (1998). "Sodium borohydride in carboxylic acid media: a phenomenal reduction system". Chemical Society Reviews. 27 (6): 395. doi:10.1039/A827395Z. S2CID 96906861.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Houri, Ahmad F.; Hoveyda, Amir H. (2001). "Tetramethylammonium Triacetoxyborohydride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt059. ISBN 0-471-93623-5.