 | |
| Identifiers | |
|---|---|
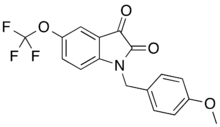
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H12F3NO4 |
| Molar mass | 351.281 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
VU-0238429 is a drug which acts as a selective positive allosteric modulator for the muscarinic acetylcholine receptor M5. It was the first selective ligand developed for the M5 subtype, and is structurally derived from older M1-selective positive allosteric modulators such as VU-0119498. Replacing the O-methyl- by a phenyl group further improves the receptor subtype selectivity.
References
- Bridges TM, Marlo JE, Niswender CM, Jones CK, Jadhav SB, Gentry PR, et al. (June 2009). "Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins". Journal of Medicinal Chemistry. 52 (11): 3445–3448. doi:10.1021/jm900286j. PMC 3875304. PMID 19438238.
- Conn PJ, Jones CK, Lindsley CW (March 2009). "Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders". Trends in Pharmacological Sciences. 30 (3): 148–155. doi:10.1016/j.tips.2008.12.002. PMC 2907736. PMID 19201489.
- Bridges TM, Kennedy JP, Hopkins CR, Conn PJ, Lindsley CW (October 2010). "Heterobiaryl and heterobiaryl ether derived M5 positive allosteric modulators". Bioorganic & Medicinal Chemistry Letters. 20 (19): 5617–5622. doi:10.1016/j.bmcl.2010.08.042. PMC 3179183. PMID 20801651.
- Bridges TM, Phillip Kennedy J, Noetzel MJ, Breininger ML, Gentry PR, Conn PJ, Lindsley CW (March 2010). "Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM". Bioorganic & Medicinal Chemistry Letters. 20 (6): 1972–1975. doi:10.1016/j.bmcl.2010.01.109. PMC 2834874. PMID 20156687.