| Revision as of 23:32, 26 November 2012 editReo On (talk | contribs)Extended confirmed users2,021 edits removed Category:Herbicides; added Category:Auxinic herbicide using HotCat← Previous edit | Latest revision as of 23:26, 25 October 2023 edit undoBenjah-bmm27 (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers14,702 edits added a ball-and-stick model of the molecule based on crystallography | ||
| (34 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| ⚫ | | Reference =<ref>''Merck Index'', 11th Edition, '''5666'''.</ref> | ||
| | verifiedrevid = 431632775 | |||
| | ImageFile =Mecoprop.svg | |||
| ⚫ | |Reference=<ref>''Merck Index'', 11th Edition, '''5666'''.</ref> | ||
| ⚫ | | ImageSize = | ||
| |ImageFile=(±)-Mecoprop Enantiomers Formulae.png | |||
| ⚫ | | ImageFile1 = Mecoprop-racemic-dimer-from-xtal-labelled-3D-balls.png | ||
| ⚫ | |ImageSize= |
||
| ⚫ | | IUPACName =(''RS'')-2-(4-Chloro-2-methylphenoxy)propanoic acid | ||
| ⚫ | |ImageFile1 = Mecoprop-racemic-dimer-from-xtal-labelled-3D-balls.png | ||
| ⚫ | | OtherNames = | ||
| |ImageSize1=250px | |||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | |IUPACName=(''RS'')-2-(4- |
||
| ⚫ | | CASNo =93-65-2 | ||
| ⚫ | |OtherNames= | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | |Section1= |
||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = 74N8TKR9P8 | |||
| ⚫ | | |
||
| | |
| PubChem =7153 | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ⚫ | | |
||
| | ChEMBL = 2145254 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C18742 | | KEGG = C18742 | ||
| | EINECS = 230-386-8 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 6886 | |||
| ⚫ | | SMILES = CC1=C(C=CC(=C1)Cl)OC(C)C(=O)O | ||
| | InChI = 1/C10H11ClO3/c1-6-5-8(11)3-4-9(6)14-7(2)10(12)13/h3-5,7H,1-2H3,(H,12,13) | |||
| | InChIKey = WNTGYJSOUMFZEP-UHFFFAOYAN | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C10H11ClO3/c1-6-5-8(11)3-4-9(6)14-7(2)10(12)13/h3-5,7H,1-2H3,(H,12,13) | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = WNTGYJSOUMFZEP-UHFFFAOYSA-N | |||
| | RTECS = | |||
| | MeSHName = | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 75704 | |||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | C=10 | H=11 | Cl=1 | O=3 | |||
| | Formula=C<sub>10</sub>H<sub>11</sub>ClO<sub>3</sub> | |||
| | Appearance =Solid | |||
| | MolarMass=214.646 | |||
| | Density = | |||
| | Appearance=Solid | |||
| | MeltingPtC = 94 to 95 | |||
| | Density= | |||
| | |
| MeltingPt_ref = <ref name="GESTIS">{{GESTIS|ZVG=510276|Name=Mecoprop|Date=8 September 2008}}</ref> | ||
| | |
| BoilingPt = decomposes <ref name="GESTIS"/> | ||
| | |
| Solubility = 900 mg/L (20 °C)<ref name="GESTIS"/> | ||
| ⚫ | |||
| ⚫ | |Section3= |
||
| ⚫ | | |
||
| | FlashPt= | |||
| | Autoignition= | |||
| }} | }} | ||
| ⚫ | |Section3={{Chembox Hazards | ||
| ⚫ | | MainHazards = Xn, N <ref name="GESTIS"/> | ||
| | FlashPt = | |||
| | AutoignitionPt = | |||
| ⚫ | }} | ||
| }} | }} | ||
| '''Mecoprop''' |
'''Mecoprop''' (also known as '''methylchlorophenoxypropionic acid''' and '''MCPP''') is a common general use ] found in many household weed killers and "weed-and-feed" type lawn fertilizers.<ref>{{CPID|id=2083|name=2-(2-Methyl-4-chlorophenoxy)propionic acid}}</ref> It is primarily used to control ].<ref name="EXTOXNET"> at EXTOXNET</ref> It is often used in combination with other chemically related herbicides such as ], ], and ], which mimic the plant hormone ] (auxin) and kill most broadleaf weeds by causing uncontrolled growth. | ||
| ⚫ | Mecoprop is a mixture of two ]s, with the (R)-(+)-] ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity<ref>{{ cite journal | journal = Acta |
||
| The ] has classified mecoprop as toxicity class III - slightly toxic.<ref name="EXTOXNET"/> | The ] has classified mecoprop as toxicity class III - slightly toxic.<ref name="EXTOXNET"/> | ||
| ⚫ | Mecoprop is a mixture of two ]s, with the (''R'')-(+)-] ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.<ref>{{ cite journal | journal = Acta Crystallogr. B| volume = 36 | issue = 4 |date=April 1980 | pages = 992–994 | doi = 10.1107/S0567740880005134 | title = (±)-2-(4-Chloro-2-methylphenoxy)propionic acid (mecoprop) |author1=G. Smith |author2=C. H. L. Kennard |author3=A. H. White |author4=P. G. Hodgson }}</ref> | ||
| ] | |||
| == See also == | == See also == | ||
| * ] | * ] | ||
| * ] | * ] | ||
| == References == | == References == | ||
| Line 47: | Line 64: | ||
| == External links == | == External links == | ||
| * | * | ||
| * {{PPDB|430|Name=Mecoprop}} | |||
| * | |||
| * {{PPDB|431|Name=Mecoprop-P}} | |||
| * | |||
| {{Herbicides}} | |||
| * | |||
| ⚫ | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 23:26, 25 October 2023
 | |
 | |
| Names | |
|---|---|
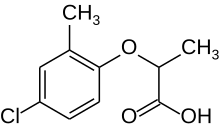
| IUPAC name (RS)-2-(4-Chloro-2-methylphenoxy)propanoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.060 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H11ClO3 |
| Molar mass | 214.65 g·mol |
| Appearance | Solid |
| Melting point | 94 to 95 °C (201 to 203 °F; 367 to 368 K) |
| Boiling point | decomposes |
| Solubility in water | 900 mg/L (20 °C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Xn, N |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Mecoprop (also known as methylchlorophenoxypropionic acid and MCPP) is a common general use herbicide found in many household weed killers and "weed-and-feed" type lawn fertilizers. It is primarily used to control broadleaf weeds. It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA, which mimic the plant hormone IAA (auxin) and kill most broadleaf weeds by causing uncontrolled growth.
The United States Environmental Protection Agency has classified mecoprop as toxicity class III - slightly toxic.
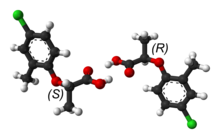
Mecoprop is a mixture of two stereoisomers, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.

See also
References
- Merck Index, 11th Edition, 5666.
- ^ Record of Mecoprop in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 8 September 2008.
- 2-(2-Methyl-4-chlorophenoxy)propionic acid in the Consumer Product Information Database
- ^ Mecoprop at EXTOXNET
- G. Smith; C. H. L. Kennard; A. H. White; P. G. Hodgson (April 1980). "(±)-2-(4-Chloro-2-methylphenoxy)propionic acid (mecoprop)". Acta Crystallogr. B. 36 (4): 992–994. doi:10.1107/S0567740880005134.
External links
- Mecoprop Pesticide Information Profile - Extension Toxicology Network
- Mecoprop in the Pesticide Properties DataBase (PPDB)
- Mecoprop-P in the Pesticide Properties DataBase (PPDB)