| Revision as of 15:18, 3 February 2013 view source92.239.18.99 (talk)No edit summary← Previous edit | Latest revision as of 22:57, 18 October 2024 view source PrimeBOT (talk | contribs)Bots2,066,063 editsm Task 24: elink template removal following a TFDTag: AWB | ||
| (944 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Region of the stratosphere}} | |||
| The '''ozone layer''' is a layer in ] containing relatively high concentrations of ] (O<sub>3</sub>). However, "relatively high," in the case of ozone, is still very small with regard to ordinary oxygen, and is less than ten parts per million, with the average ozone concentration in Earth's atmosphere being only about 0.6 parts per million. The ozone layer is mainly found in the lower portion of the ] from approximately {{convert|20|to|30|km}} above Earth, though the thickness varies seasonally and geographically.<ref>{{cite web|url=http://www.ozonelayer.noaa.gov/science/basics.htm|title=Science: Ozone Basics.|accessdate=2007-01-29}}</ref>i like poo | |||
| {{pp-semi-indef}} | |||
| {{Use mdy dates|date=August 2023}} | |||
| ] within the bottom of the large bright blue band that is the ], with a silhouette of a ] in the orange afterglow of the ].]] | |||
| The '''ozone layer''' or '''ozone shield''' is a region of ]'s ] that ] most of the ]'s ] radiation. It contains a high concentration of ] (O<sub>3</sub>) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 ] of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately {{convert|15|to|35|km|sp=us|0}} above Earth, although its thickness varies seasonally and geographically.<ref>{{cite web|url=http://www.ozonelayer.noaa.gov/science/basics.htm|title=Ozone Basics|website=NOAA|date=March 20, 2008|access-date=January 29, 2007|archive-url=https://web.archive.org/web/20171121051325/http://www.ozonelayer.noaa.gov/science/basics.htm|archive-date=November 21, 2017|url-status=dead}}</ref> | |||
| One direction were discovered in 1913 by the Irish/English physicists ] and ]. Its properties were explored in detail by the British meteorologist ], who developed a simple ] (the ]) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958 Tomlinson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The "]", a convenient measure of the ] of ozone overhead, is named in his honor. | |||
| The ozone layer was discovered in 1913 by French physicists ] and ]. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the ] of a ] with a temperature in the range of {{convert|5500|–|6000|K|C}}, except that there was no radiation below a ] of about 310 nm at the ] end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone.<ref>{{Cite journal|journal=Atmosphere-Ocean|volume=46|pages=1–13|doi=10.3137/ao.460101|year=2008|last1=McElroy|first1=C.T.|title=Ozone: From discovery to protection|last2=Fogal|first2=P.F.|issue=1 |bibcode=2008AtO....46....1M |s2cid=128994884}}</ref> Its properties were explored in detail by the British ] ], who developed a simple ] (the ]) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The "]" (DU), a convenient measure of the ] of ozone overhead, is named in his honor. | |||
| The ozone layer absorbs 97–99% of the ]'s medium-frequency ] (from about 200 nm to 315 nm wavelength), which potentially damages exposed life forms on Earth.<ref name="NASA">{{cite web|url=http://www.nas.nasa.gov/About/Education/Ozone/ozonelayer.html|title=Ozone layer|accessdate=2007-09-23}}</ref> | |||
| The ozone layer absorbs 97 to 99 percent of the Sun's medium-frequency ultraviolet light (from about 200 ] to 315 nm ]), which otherwise would potentially damage exposed life forms near the surface.<ref name="NASA">{{cite web|url=http://www.nas.nasa.gov/About/Education/Ozone/ozonelayer.html|title=Ozone layer|access-date=September 23, 2007|archive-date=May 2, 2021|archive-url=https://web.archive.org/web/20210502050928/https://www.nas.nasa.gov/About/Education/Ozone/ozonelayer.html|url-status=dead}}</ref> | |||
| ] in the ozone layer.]] | |||
| ==Origin of ozone== | |||
| The photochemical mechanisms that give rise to the ozone layer were discovered by the British physicist ] in 1930. Ozone in the Earth's stratosphere is created by ] striking ] ]s containing two oxygen ]s (O<sub>2</sub>), splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O<sub>2</sub> to create ozone, O<sub>3</sub>. The ozone molecule is also unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O<sub>2</sub> and an atom of atomic oxygen, a continuing process called the ], thus creating an ozone layer in the ], the region from about {{convert|10|to|50|km|ft}} above Earth's surface. About 90% of the ozone in our atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about {{convert|20|and|40|km}}, where they range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of the air at sea level, it would be only 3 millimeters thick.<ref>{{cite web|url=http://www.nasa.gov/facts/Earth/earth_facts_archives.html|title=NASA Facts Archive|accessdate=2011-06-09}}</ref> | |||
| In 1985, atmospheric research revealed that the ozone layer was being depleted by chemicals released by industry, mainly ] (CFCs). Concerns that increased UV radiation due to ] threatened life on Earth, including increased skin cancer in humans and other ecological problems,<ref>An Interview with Lee Thomas, EPA's 6th Administrator. , (see p13). April 19, 2012.</ref> led to bans on the chemicals, and the latest evidence is that ozone depletion has slowed or stopped. The United Nations General Assembly has designated September 16 as the ]. | |||
| ==Ultraviolet light and ozone== | |||
| ] | |||
| ] of dioxygen (below 240 nm) react with more dioxygen. The ozone layer also blocks most, but not quite all, of the sunburn-producing UV-B (280–315 nm) band, which lies in the wavelengths longer than UV-C. The band of UV closest to visible light, UV-A (315–400 nm), is hardly affected by ozone, and most of it reaches the ground. UVA does not cause skin reddening, but there is evidence that it causes long-term skin damage.]] | |||
| Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation coming from the sun. Extremely short or vacuum UV (10–100 nm) is screened out by nitrogen. UV radiation capable of penetrating nitrogen is divided into three categories, based on its ]; these are referred to as UV-A (400–315 ]), UV-B (315–280 nm), and UV-C (280–100 nm). | |||
| ] also has a thin ozone layer at an altitude of 100 kilometers above the planet's surface.<ref name="venus ozone">{{cite web | url=http://www.space.com/13244-venus-atmosphere-ozone-layer.html | title=Scientists discover Ozone Layer on Venus | publisher=Purch | work=SPACE.com | date=October 11, 2011 | access-date=October 3, 2015 | author=SPACE.com staff}}</ref> | |||
| UV-C, which would be very harmful to all living things, is entirely screened out by a combination of dioxygen (< 200 nm) and ozone (> about 200 nm) by around {{convert|35|km|ft}} altitude. UV-B radiation can be harmful to the skin and is the main cause of ]; excessive exposure can also cause genetic damage, resulting in problems such as ]. The ozone layer (which absorbs from about 200 nm to 310 nm with a maximal absorption at about 250 nm) <ref> See the graphical absorption of ozone in two bands, as a function of wavelength</ref> is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at the top of the atmosphere is 350 million times stronger than at the Earth's surface. Nevertheless, some UV-B, particularly at its longest wavelengths, reaches the surface. | |||
| ==Sources== | |||
| Ozone is transparent to most UV-A, so most of this type of longer wavelength reaches the surface, and becomes the primary type of UV from the Sun to do so. However, this type of UV radiation is significantly less harmful to DNA, although it may still potentially cause indirect genetic damage in skin (see ] for more about UV-A). | |||
| {{Main|Ozone–oxygen cycle}} | |||
| ] in the ozone layer]] | |||
| The ] mechanisms that give rise to the ozone layer were discovered by the British physicist ] in 1930. Ozone in the Earth's stratosphere is created by ultraviolet light striking ordinary ] ]s containing two oxygen ]s (O<sub>2</sub>), splitting them into individual oxygen atoms (]); the atomic oxygen then combines with unbroken O<sub>2</sub> to create ozone, O<sub>3</sub>. The ozone molecule is unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O<sub>2</sub> and an individual atom of oxygen, a continuing process called the ]. Chemically, this can be described as: | |||
| : <chem>O2{} + \mathit{h}\nu_{uv} -> 2O </chem> | |||
| : <chem>O + O2 <-> O3</chem> | |||
| About 90 percent of the ozone in the atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about {{convert|20|and|40|km|ft}}, where they range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of the air at sea level, it would be only {{convert|3|mm|in|abbr=off|frac=8}} thick.<ref>{{cite web|url=http://www.nasa.gov/facts/Earth/earth_facts_archives.html|title=NASA Facts Archive|access-date=June 9, 2011|archive-date=April 6, 2013|archive-url=https://web.archive.org/web/20130406065500/http://www.nasa.gov/facts/Earth/earth_facts_archives.html|url-status=dead}}</ref> | |||
| ==Ultraviolet light== | |||
| ==Distribution of ozone in the stratosphere== | |||
| ] | |||
| {{unreferencedsection|date=February 2013}} | |||
| ] of dioxygen (below 240 nm) react with more dioxygen. The ozone layer also blocks most, but not quite all, of the sunburn-producing ] (280–315 nm) band, which lies in the wavelengths longer than UV-C. The band of UV closest to visible light, ] (315–400 nm), is hardly affected by ozone, and most of it reaches the ground. UV-A does not primarily cause skin reddening, but there is evidence that it causes long-term skin damage.]] | |||
| Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation coming from the Sun. Extremely short or vacuum UV (10–100 nm) is screened out by nitrogen. UV radiation capable of penetrating nitrogen is divided into three categories, based on its wavelength; these are referred to as UV-A (400–315 nm), ] (315–280 nm), and ] (280–100 nm). | |||
| The thickness of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, being in general smaller near the equator and larger towards the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn in the northern hemisphere. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity. | |||
| UV-C, which is very harmful to all living things, is entirely screened out by a combination of dioxygen (< 200 nm) and ozone (> about 200 nm) by around {{convert|35|km|ft}} altitude. UV-B radiation can be harmful to the skin and is the main cause of ]; excessive exposure can also cause cataracts, immune system suppression, and genetic damage, resulting in problems such as ]. The ozone layer (which absorbs from about 200 nm to 310 nm with a maximal absorption at about 250 nm)<ref>{{cite journal|url=http://yly-mac.gps.caltech.edu/N2O/Prasad/Matsumi_O3_cr0205255%20copy.pdf |title=Photolysis of Atmospheric Ozone in the Ultraviolet Region |author1=Matsumi, Y. |author2=Kawasaki, M. |journal=Chem. Rev. |date=2003 |volume=103 |issue=12 |pages=4767–4781 |pmid=14664632 |access-date=March 14, 2015 |doi=10.1021/cr0205255 |url-status=dead |archive-url=https://web.archive.org/web/20120617123007/http://yly-mac.gps.caltech.edu/N2O/Prasad/Matsumi_O3_cr0205255%20copy.pdf |archive-date=June 17, 2012 }}</ref> is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at the top of the atmosphere is 350 million times stronger than at the Earth's surface. Nevertheless, some UV-B, particularly at its longest wavelengths, reaches the surface, and is important for the skin's production of ] in ]. | |||
| Since stratospheric ozone is produced by solar UV radiation, one might expect to find the highest ozone levels over the tropics and the lowest over polar regions. The same argument would lead one to expect the highest ozone levels in the summer and the lowest in the winter. The observed behavior is very different: most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres, and the highest levels are found in the spring, not summer, and the lowest in the autumn, not winter in the northern hemisphere. During winter, the ozone layer actually increases in depth. This puzzle is explained by the prevailing stratospheric wind patterns, known as the ]. While most of the ozone is indeed created over the tropics, the stratospheric circulation then transports it poleward and downward to the lower stratosphere of the high latitudes. However in the southern hemisphere, owing to the ] phenomenon, the lowest amounts of column ozone found anywhere in the world are over the Antarctic in the southern spring period of September and October. | |||
| Ozone is transparent to most UV-A, so most of this longer-wavelength UV radiation reaches the surface, and it constitutes most of the UV reaching the Earth. This type of UV radiation is significantly less harmful to ], although it may still potentially cause physical damage, premature aging of the skin, indirect genetic damage, and skin cancer.<ref>{{cite journal |title=Review: Ultraviolet radiation and skin cancer |author1=Narayanan, D.L. |author2=Saladi, R.N. |author3=Fox, J.L. |journal=International Journal of Dermatology |volume=49 |issue=9 |pages=978–986 |date=2010|pmid=20883261 |doi=10.1111/j.1365-4632.2010.04474.x |s2cid=22224492 |doi-access=free }}</ref> | |||
| ] | |||
| ==Distribution in the stratosphere== | |||
| The ozone layer is higher in altitude in the tropics, and lower in altitude in the extratropics, especially in the polar regions. This altitude variation of ozone results from the slow circulation that lifts the ozone-poor air out of the troposphere into the stratosphere. As this air slowly rises in the tropics, ozone is produced by the overhead sun which photolyzes oxygen molecules. As this slow circulation bends towards the mid-latitudes, it carries the ozone-rich air from the tropical middle stratosphere to the mid-and-high latitudes lower stratosphere. The high ozone concentrations at high latitudes are due to the accumulation of ozone at lower altitudes. | |||
| {{more citations needed section|date=February 2013}} | |||
| ] | |||
| The thickness of the ozone layer varies worldwide and is generally thinner near the equator and thicker near the poles.<ref name="APH">{{cite book|url=https://books.google.com/books?id=QFBmUu1lwzAC|title=Global Warming: The Effect Of Ozone Depletion|author=Tabin, Shagoon|publisher=APH Publishing|year=2008|isbn=9788131303962|page=194|access-date=January 12, 2016}}</ref> Thickness refers to how much ozone is in a column over a given area and varies from season to season. The reasons for these variations are due to atmospheric circulation patterns and solar intensity.<ref>{{Cite web|title=Nasa Ozone Watch: Ozone facts|url=https://ozonewatch.gsfc.nasa.gov/facts/SH.html|access-date=September 16, 2021|website=ozonewatch.gsfc.nasa.gov}}</ref> | |||
| The Brewer-Dobson circulation moves very slowly. The time needed to lift an air parcel from the tropical tropopause near {{convert|16|to|20|km}} is about 4–5 months (about {{convert|30|ft|m}} per day). Even though ozone in the lower tropical stratosphere is produced at a very slow rate, the lifting circulation is so slow that ozone can build up to relatively high levels by the time it reaches {{convert|26|km}}. | |||
| The majority of ozone is produced over the ] and is transported towards the poles by stratospheric wind patterns. In the northern hemisphere these patterns, known as the ], make the ozone layer thickest in the spring and thinnest in the fall.<ref name="APH" /> When ozone is produced by solar UV radiation in the tropics, it is done so by circulation lifting ozone-poor air out of the troposphere and into the stratosphere where the sun ] oxygen molecules and turns them into ozone. Then, the ozone-rich air is carried to higher latitudes and drops into lower layers of the atmosphere.<ref name="APH" /> | |||
| Ozone amounts over the continental ] (25°N to 49°N) are highest in the northern spring (April and May). These ozone amounts fall over the course of the summer to their lowest amounts in October, and then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal evolution of these higher latitude ozone patterns. | |||
| Research has found that the ozone levels in the United States are highest in the spring months of April and May and lowest in October. While the total amount of ozone increases moving from the tropics to higher latitudes, the concentrations are greater in high northern latitudes than in high southern latitudes, with spring ozone columns in high northern latitudes occasionally exceeding 600 DU and averaging 450 DU whereas 400 DU constituted a usual maximum in the Antarctic before anthropogenic ozone depletion. This difference occurred naturally because of the weaker polar vortex and stronger Brewer–Dobson circulation in the northern hemisphere owing to that hemisphere's large mountain ranges and greater contrasts between land and ocean temperatures.<ref>{{cite journal|author=Douglass|first1=Anne R.|last2=Newman|first2=Paul A.|last3=Solomon|first3=Susan|title=The Antarctic ozone hole: An update|journal=Physics Today|year=2014|volume=67|issue=7|pages=42–48|url=https://physicstoday.scitation.org/doi/pdf/10.1063/PT.3.2449|publisher=American Institute of Physics|doi=10.1063/PT.3.2449|bibcode=2014PhT....67g..42D|hdl=1721.1/99159|hdl-access=free}}</ref> The difference between high northern and southern latitudes has increased since the 1970s due to the ] phenomenon.<ref name="APH" /> The highest amounts of ozone are found over the Arctic during the spring months of March and April, but the Antarctic has the lowest amounts of ozone during the summer months of September and October, | |||
| The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. In addition, while the highest amounts of column ozone over the Arctic occur in the northern spring (March–April), the opposite is true over the Antarctic, where the lowest amounts of column ozone occur in the southern spring (September–October). | |||
| ] | |||
| ==Ozone depletion== | |||
| ==Depletion== | |||
| {{Main|Ozone depletion}} | {{Main|Ozone depletion}} | ||
| ] | ]s had not been banned]] | ||
| The ozone layer can be depleted by free radical catalysts, including ] (NO), ] (N<sub>2</sub>O), ] (OH), atomic ] (Cl), and atomic ] (Br). While there are natural sources for all of these ], the concentrations of chlorine and bromine have increased markedly in recent years due to the release of large quantities of man-made ] compounds, especially ]s (CFCs) and ].<ref>{{cite web|url=http://www.eia.doe.gov/oiaf/1605/archive/gg97rpt/chap5.html|title=Halocarbons and Other Gases|accessdate=2008-06-24|author=Energy Information Administration/Emissions of Greenhouse Gases in the United States 1996|date=2008-06-24}}</ref> These highly stable compounds are capable of surviving the rise to the ], where Cl and Br ] are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 100,000 ozone molecules. The breakdown of ozone in the stratosphere results in the ozone molecules being unable to absorb ultraviolet radiation. Consequently, unabsorbed and dangerous ultraviolet-B radiation is able to reach the Earth’s surface. Ozone levels over the ] have been dropping by 4% per decade. Over approximately 5% of the Earth's surface, around the north and south poles, much larger seasonal declines have been seen, and are described as ]s. | |||
| The ozone layer can be depleted by ] ], including ] (NO), ] (N<sub>2</sub>O), ] (OH), atomic ] (Cl), and atomic ] (Br). While there are natural sources for all of these ], the concentrations of chlorine and bromine increased markedly in recent decades because of the release of large quantities of man-made ] compounds, especially ]s (CFCs) and ].<ref>{{cite book |chapter-url=http://www.eia.doe.gov/oiaf/1605/archive/gg97rpt/chap5.html |chapter=Halocarbons and Other Gases |publisher=Energy Information Administration |title=Emissions of Greenhouse Gases in the United States 1996 |date=1997 |access-date=June 24, 2008 |url-status=dead |archive-url=https://web.archive.org/web/20080629032506/http://www.eia.doe.gov/oiaf/1605/archive/gg97rpt/chap5.html |archive-date=June 29, 2008 }}</ref> These highly stable compounds are capable of surviving the rise to the ], where Cl and Br ] are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 100,000 ozone molecules. By 2009, nitrous oxide was the largest ozone-depleting substance (ODS) emitted through human activities.<ref>{{cite web |url=http://www.noaanews.noaa.gov/stories2009/20090827_ozone.html |title=NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission |publisher=NOAA |date=August 27, 2009 |access-date=November 8, 2011}}</ref> | |||
| In 2009, ] (N<sub>2</sub>O) was the largest ozone-depleting substance emitted through human activities.<ref>{{cite web|url=http://www.noaanews.noaa.gov/stories2009/20090827_ozone.html |title=NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission, NOAA, August 27, 2009 |publisher=Noaanews.noaa.gov |date=2009-08-27 |accessdate=2011-11-08}}</ref> | |||
| The breakdown of ozone in the stratosphere results in reduced absorption of ultraviolet radiation. Consequently, unabsorbed and dangerous ultraviolet radiation is able to reach the Earth's surface at a higher intensity. Ozone levels have dropped by a worldwide average of about 4 percent since the late 1970s. For approximately 5 percent of the Earth's surface, around the north and south poles, much larger seasonal declines have been seen, and are described as "ozone holes". "Ozone holes" are actually patches in the ozone layer in which the ozone is thinner. The thinnest parts of the ozone are at the ].<ref>{{Cite web |title=ozone layer {{!}} National Geographic Society |url=https://education.nationalgeographic.org/resource/ozone-layer |access-date=May 30, 2022 |website=education.nationalgeographic.org}}</ref> The discovery of the annual depletion of ozone above the Antarctic was first announced by ], ] and ], in a paper which appeared in '']'' on May 16, 1985. | |||
| ===Regulation=== | |||
| In 1978, the ], ] and ] enacted bans on ]-containing ]s that are thought to damage the ozone layer. The European Community rejected an analogous proposal to do the same. In the U.S., chlorofluorocarbons continued to be used in other applications, such as refrigeration and industrial cleaning, until after the discovery of the Antarctic ] in 1985. After negotiation of an international treaty (the ]), CFC production was sharply limited beginning in 1987 and phased out completely by 1996.{{Citation needed|date=November 2010}} Since that time, the treaty has been amended to ban CFC production after 1995 in the developed countries, and later in developing. Today, over 160 countries have signed the treaty. Beginning January 1, 1996, only recycled and stockpiled CFCs will be available for use in developed countries like the US. This production phaseout is possible because of efforts to ensure that there will be substitute chemicals and technologies for all CFC uses.<ref>{{cite web|url=http://www.epa.gov/ozone/science/q_a.html |title=Brief Questions and Answers on Ozone Depletion | Ozone Layer Protection | US EPA |publisher=Epa.gov |date=2006-06-28 |accessdate=2011-11-08}}</ref> | |||
| Regulation attempts have included but not have been limited to the ] implemented by the ]. The Clean Air Act introduced the requirement of with ozone pollutions being one of six criteria pollutants. This regulation has proven to be effective since counties, cities and tribal regions must abide by these standards and the EPA also provides assistance for each region to regulate contaminants.<ref>{{Cite web |last=US EPA |first=OAR |date=December 14, 2016 |title=Ozone Implementation Regulatory Actions |url=https://www.epa.gov/ground-level-ozone-pollution/ozone-implementation-regulatory-actions |access-date=May 30, 2022 |website=www.epa.gov |language=en}}</ref> Effective presentation of information has also proven to be important in order to educate the general population of the existence and regulation of ozone depletion and contaminants. A scientific paper was written by Sheldon Ungar in which the author explores and studies how information about the depletion of the ozone, ] and various related topics. The ozone case was communicated to lay persons "with easy-to-understand bridging metaphors derived from the popular culture" and related to "immediate risks with everyday relevance".<ref>{{Cite journal |last=Ungar |first=Sheldon |date=July 2000 |title=Knowledge, ignorance and the popular culture: climate change versus the ozone hole |url=http://journals.sagepub.com/doi/10.1088/0963-6625/9/3/306 |journal=Public Understanding of Science |language=en |volume=9 |issue=3 |pages=297–312 |doi=10.1088/0963-6625/9/3/306 |s2cid=7089937 |issn=0963-6625}}</ref> The specific metaphors used in the discussion (ozone shield, ozone hole) proved quite useful and, compared to global climate change, the ozone case was much more seen as a "hot issue" and imminent risk. Lay people were cautious about a depletion of the ozone layer and the risks of skin cancer. | |||
| On August 2, 2003, scientists announced that the depletion of the ozone layer may be slowing down due to the international ban on CFCs.<ref>{{cite news |url=http://www.ecozine.co.uk/OzoneLayer.htm|title=Ozone_Layer. |accessdate=2010-11-09 |author=EcoZone |date=2005-01-01 |archiveurl = http://web.archive.org/web/20060830060353/http://www.ecozine.co.uk/OzoneLayer.htm <!-- Bot retrieved archive --> |archivedate = 2010-11-09 | location=unknown | work=ecozone}}</ref> Three satellites and three ground stations confirmed that the upper atmosphere ozone depletion rate has slowed down significantly during the past decade. The study was organized by the ]. Some breakdown can be expected to continue due to CFCs used by nations which have not banned them, and due to gases which are already in the stratosphere. CFCs have very long atmospheric lifetimes, ranging from 50 to over 100 years. It has been estimated that the ozone layer may not recover until 2075.<ref>. Phys.org (September 16, 2009). Retrieved on 2013-01-17.</ref> | |||
| ]s burning up upon re-entry into Earth's atmosphere produce ] (Al<sub>2</sub>O<sub>3</sub>) ]s that endure in the atmosphere for decades.<ref name=GeophysResearchLtrs_20240611/> Estimates for 2022 alone were ~17 metric tons (~30{{nbsp}}kg of nanoparticles per ~250{{nbsp}}kg satellite).<ref name=GeophysResearchLtrs_20240611/> Increasing populations of ]s can eventually lead to significant ozone depletion.<ref name=GeophysResearchLtrs_20240611>{{cite journal |last1=Ferreira |first1=Jose P. |last2=Huang |first2=Ziyu |last3=Nomura |first3=Ken-ichi |last4=Wang |first4=Joseph |title=Potential Ozone Depletion From Satellite Demise During Atmospheric Reentry in the Era of Mega-Constellations |journal=Geophysical Research Letters |date=11 June 2024 |doi=10.1029/2024GL109280|doi-access=free }}</ref> | |||
| Compounds containing C–H bonds (such as ]s, or HCFCs) have been designed to replace the function of CFCs. These replacement compounds are more reactive and less likely to survive long enough in the atmosphere to reach the stratosphere where they could affect the ozone layer. While being less damaging than CFCs, HCFCs can have a negative impact on the ozone layer, so they are also being phased out.<ref>{{cite web|url=http://www.epa.gov/ozone/defns.html#hcfc|title=Ozone Depletion Glossary|accessdate=2008-09-03|author=US EPA|date=2008-09-03}}</ref> | |||
| ] can cause adverse health risks respiratory effects (difficulty breathing) and is proven to be an aggravator of respiratory illnesses such as ], ] and ].<ref>{{Cite journal |last1=Zhang |first1=Junfeng (Jim) |last2=Wei |first2=Yongjie |last3=Fang |first3=Zhangfu |date=2019 |title=Ozone Pollution: A Major Health Hazard Worldwide |journal=Frontiers in Immunology |volume=10 |page=2518 |doi=10.3389/fimmu.2019.02518 |pmid=31736954 |pmc=6834528 |issn=1664-3224|doi-access=free }}</ref> That is why many countries have set in place regulations to improve "good" ozone and prevent the increase of "bad" ozone in urban or residential areas. In terms of ozone protection (the preservation of "good" ozone) the ] has strict guidelines on what products are allowed to be bought, distributed or used in specific areas.<ref>{{Cite web |title=Ozone Regulation |url=https://ec.europa.eu/clima/eu-action/protection-ozone-layer/ozone-regulation_en |access-date=May 30, 2022 |website=ec.europa.eu |language=en}}</ref> With effective regulation, the ozone is expected to heal over time.<ref>{{Cite web |last=US EPA |first=OAR |date=July 15, 2015 |title=International Treaties and Cooperation about the Protection of the Stratospheric Ozone Layer |url=https://www.epa.gov/ozone-layer-protection/international-treaties-and-cooperation-about-protection-stratospheric-ozone |access-date=May 30, 2022 |website=www.epa.gov |language=en}}</ref>] | |||
| {{main|Ozone depletion and climate change}} | |||
| In 1978, the United States, Canada and ] enacted bans on ]-containing ]s that damage the ozone layer but the European Community rejected a similar proposal. In the U.S., chlorofluorocarbons continued to be used in other applications, such as refrigeration and industrial cleaning, until after the discovery of the Antarctic ozone hole in 1985. After negotiation of an international treaty (the ]), CFC production was capped at 1986 levels with commitments to long-term reductions.<ref>{{cite journal |title=The Evolution of Policy Responses to Stratospheric Ozone Depletion |journal=Natural Resources Journal |date=1989 |first=Peter M. |last=Morrisette |volume=29 |pages=793–820 |url=http://www.ciesin.org/docs/003-006/003-006.html |access-date=April 20, 2010}}</ref> This allowed for a ten-year phase-in for developing countries<ref>An Interview with Lee Thomas, EPA's 6th Administrator. , (see p15). April 19, 2012.</ref> (identified in Article 5 of the protocol). Since then, the treaty was amended to ban CFC production after 1995 in developed countries, and later in developing countries.<ref>{{cite web |url=http://www.epa.gov/ozone/intpol/history.html |title=Amendments to the Montreal Protocol |publisher=EPA |date=August 19, 2010 |access-date=March 28, 2011}}</ref> All of the world's 197 countries have signed the treaty. Beginning January 1, 1996, only recycled or stockpiled CFCs were available for use in developed countries like the US. The production phaseout was possible because of efforts to ensure that there would be substitute chemicals and technologies for all ODS uses.<ref>{{cite web |url=http://www.epa.gov/ozone/science/q_a.html |title=Brief Questions and Answers on Ozone Depletion |publisher=EPA |date=June 28, 2006 |access-date=November 8, 2011}}</ref> | |||
| On August 2, 2003, scientists announced that the global depletion of the ozone layer might be slowing because of the international regulation of ozone-depleting substances. In a study organized by the ], three satellites and three ground stations confirmed that the upper-atmosphere ozone-depletion rate slowed significantly over the previous decade. Some breakdown was expected to continue because of ODSs used by nations which have not banned them, and because of gases already in the stratosphere. Some ODSs, including CFCs, have very long atmospheric lifetimes ranging from 50 to over 100 years. It has been estimated that the ozone layer will recover to 1980 levels near the middle of the 21st century.<ref name="wmo2010">{{cite book |title=Scientific Assessment of Ozone Depletion: 2010 |date=2011 |publisher=WMO |chapter=Stratospheric Ozone and Surface Ultraviolet Radiation |access-date=March 14, 2015 |chapter-url=http://acdb-ext.gsfc.nasa.gov/Documents/O3_Assessments/Docs/WMO_2010/Chapter_2.pdf}}</ref> A gradual trend toward "healing" was reported in 2016.<ref name=healing>{{cite journal |title=Emergence of healing in the Antarctic ozone layer |vauthors=Solomon, Susan etal |journal=Science |volume=353 |issue=6296 |pages=269–74 |date=June 30, 2016 |doi=10.1126/science.aae0061|pmid=27365314 |bibcode=2016Sci...353..269S |doi-access=free |hdl=1721.1/107197 |hdl-access=free }}</ref> | |||
| Compounds containing ] (such as ]s, or HCFCs) have been designed to replace CFCs in certain applications. These replacement compounds are more reactive and less likely to survive long enough in the atmosphere to reach the stratosphere where they could affect the ozone layer. While being less damaging than CFCs, HCFCs can have a negative impact on the ozone layer, so they are also being phased out.<ref>{{cite web |url=http://www.epa.gov/ozone/defns.html#hcfc |title=Ozone Depletion Glossary |publisher=EPA |access-date=September 3, 2008}}</ref> These in turn are being replaced by ] (HFCs) and other compounds that do not destroy stratospheric ozone at all. | |||
| The residual effects of CFCs accumulating within the atmosphere lead to a concentration gradient between the atmosphere and the ocean. This organohalogen compound is able to dissolve into the ocean's surface waters and is able to act as a ]. This tracer helps scientists study ocean circulation by tracing biological, physical and chemical pathways.<ref>{{Cite journal|url=http://yyy.rsmas.miami.edu/groups/cfc/pubs/Fine_AnnRevMarineSci3_2011.pdf|title=Observations of CFCs and SF6 as Ocean Tracers|last=Fine|first=Rana A.|date=2011|pmid=21329203|journal=Annual Review of Marine Science|volume=3|pages=173–95|doi=10.1146/annurev.marine.010908.163933|bibcode=2011ARMS....3..173F|archive-url=https://web.archive.org/web/20150210212306/http://yyy.rsmas.miami.edu/groups/cfc/pubs/Fine_AnnRevMarineSci3_2011.pdf|archive-date=February 10, 2015|url-status=dead}}</ref> | |||
| ==Implications for astronomy== | |||
| As ozone in the atmosphere prevents most energetic ultraviolet radiation reaching the surface of the Earth, astronomical data in these wavelengths have to be gathered from satellites orbiting above the atmosphere and ozone layer. Most of the light from young hot stars is in the ultraviolet and so study of these wavelengths is important for studying the origins of galaxies. The Galaxy Evolution Explorer, ], is an orbiting ultraviolet space telescope launched on April 28, 2003, which operated until early 2012.<ref>{{Cite web|date=May 9, 2011|title=ozone layer|url=http://www.nationalgeographic.org/encyclopedia/ozone-layer/|access-date=September 16, 2021|website=National Geographic Society|language=en}}</ref> | |||
| <gallery class="center" widths="300" heights="260"> | |||
| File:Ultraviolet image of the Cygnus Loop Nebula crop.jpg|This ] image of the ] could not have been taken from the surface of the Earth because the ozone layer blocks the ultra-violet radiation emitted by the nebula. | |||
| </gallery> | |||
| ==See also== | ==See also== | ||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| ==References== | ==References== | ||
| {{Reflist| |
{{Reflist|33em}} | ||
| ==Further reading== | ==Further reading== | ||
| ; Science | |||
| * Sei John H.; Pandis, Spyros N. (1998). ''Atmospheric Chemistry and Physics: From Air Pollution to Climate Change''. John Wiley and Sons, Inc. ISBN 0-471-17816-0. | |||
| * {{cite journal |authorlink=Stephen O. Andersen |last=Andersen |first=S. O. |title=Lessons from the stratospheric ozone layer protection for climate |journal=Journal of Environmental Studies and Sciences |year=2015 |volume=5 |issue=2 |pages=143–162 |doi=10.1007/s13412-014-0213-9|bibcode=2015JEnSS...5..143A |s2cid=129725437 |url=https://link.springer.com/article/10.1007/s13412-014-0213-9 }} | |||
| * {{cite book | last1=Andersen | first1=S.O. | last2=Sarma | first2=K.M. | last3=Sinclair | first3=L. | title=Protecting the Ozone Layer: The United Nations History | publisher=Taylor & Francis | year=2012 | isbn=978-1-84977-226-6 | url=https://books.google.com/books?id=zuesUPcIOq8C }} | |||
| * ], "What We Learned from Acid Rain: By working together, the nations of the world can solve climate change", '']'', vol. 330, no. 1 (January 2024), pp. 75–76. "ountries will act only if they know others are willing to do the same. With ], they did act collectively.... We did something similar to restore Earth's protective ozone layer.... he cost of technology really matters.... In the past decade the price of ] has fallen by more than 90 percent and that of ] by more than 70 percent. ] costs have tumbled by 98 percent since 1990, bringing the price of ]s down with them....he stance of ]s matters more than their ] affiliation.... Change can happen – but not on its own. We need to drive it." (p. 76.) | |||
| * ] (2010). ''Environmental Effects of Ozone Depletion and its Interactions with Climate Change: 2010 Assessment''. Nairobi: UNEP. | |||
| * {{cite journal |doi=10.1073/pnas.0902817106 |title=The large contribution of projected HFC emissions to future climate forcing |year=2009 |last1=Velders |first1=G. J. M. |last2=Fahey |first2=D. W. |last3=Daniel |first3=J. S. |last4=McFarland |first4=M. |last5=Andersen |first5=S. O. |journal=Proceedings of the National Academy of Sciences |volume=106 |issue=27 |pages=10949–10954 |pmid=19549868 |pmc=2700150 |bibcode=2009PNAS..10610949V |s2cid=3743609 |doi-access=free }} | |||
| * {{cite journal | last1 = Velders | first1 = Guus J.M. | last2 = Andersen | first2 = Stephen O. | last3 = Daniel | first3 = John S. | last4 = Fahey | first4 = David W. | last5 = McFarland | first5 = Mack | year = 2007 | title = The Importance of the Montreal Protocol in Protecting Climate | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 104 | issue = 12| pages = 4814–4819 | doi=10.1073/pnas.0610328104 | pmid=17360370|bibcode = 2007PNAS..104.4814V | pmc=1817831| doi-access = free }} | |||
| ; Policy | |||
| * {{cite journal |url=https://link.springer.com/article/10.1007%2Fs13412-014-0215-7 |doi=10.1007/s13412-014-0215-7 |title=The importance of phasing down hydrofluorocarbons and other short-lived climate pollutants |year=2015 |last1=Zaelke |first1=Durwood |last2=Borgford-Parnell |first2=Nathan |journal=Journal of Environmental Studies and Sciences |volume=5 |issue=2 |pages=169–175 |bibcode=2015JEnSS...5..169Z |s2cid=128974741 }} | |||
| * {{cite journal |doi=10.5194/acp-13-6083-2013 |url=https://acp.copernicus.org/articles/13/6083/2013/acp-13-6083-2013.html |title=The role of HFCS in mitigating 21st century climate change |year=2013 |last1=Xu |first1=Y. |last2=Zaelke |first2=D. |last3=Velders |first3=G. J. M. |last4=Ramanathan |first4=V. |journal=Atmospheric Chemistry and Physics |volume=13 |issue=12 |pages=6083–6089 |bibcode=2013ACP....13.6083X |doi-access=free }} | |||
| * {{cite journal |doi=10.1073/pnas.0902568106 |title=Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions |year=2009 |last1=Molina |first1=M. |last2=Zaelke |first2=D. |last3=Sarma |first3=K. M. |last4=Andersen |first4=S. O. |last5=Ramanathan |first5=V. |last6=Kaniaru |first6=D. |journal=Proceedings of the National Academy of Sciences |volume=106 |issue=49 |pages=20616–20621 |pmid=19822751 |pmc=2791591 |s2cid=13240115 |doi-access=free }} | |||
| * {{cite book |last1=Anderson |first1=S. O. |last2=Sarma |first2=M. K. |last3=Taddonio |first3=K. |year=2007 |url=https://books.google.com/books?id=OvgA-hZrPOcC |title=Technology Transfer for the Ozone Layer: Lessons for Climate Change |location=London |publisher=Earthscan |isbn=9781849772846 }} | |||
| * {{cite book |first1=Richard Elliot |last1=Benedick |author2=World Wildlife Fund (U.S.) |author3=Institute for the Study of Diplomacy. Georgetown University. |title=Ozone Diplomacy: New Directions in Safeguarding the Planet |edition=2nd |url=https://books.google.com/books?id=4yM9uPRUvi4C |year=1998 |publisher=Harvard University Press |isbn=978-0-674-65003-9}} (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.) | |||
| * {{cite book |last1=Chasek |first1=P. S. |first2=David L. |last2=Downie |last3=Brown |first3=J. W. |year=2013 |url=https://books.google.com/books?id=Ju41zgEACAAJ |title=Global Environmental Politics |edition=6th |location=Boulder |publisher=Westview Press |isbn=9780813348971 }} | |||
| * {{cite book |first=Reiner |last=Grundmann |title=Transnational Environmental Policy: Reconstructing Ozone |url=https://books.google.com/books?id=FYyVDlRhBvEC |year=2001 |publisher=Psychology Press |isbn=978-0-415-22423-9}} | |||
| * {{cite book |last=Parson |first=E. |year=2003 |url=https://books.google.com/books?id=VNkJCAAAQBAJ |title=Protecting the Ozone Layer: Science and Strategy |location=Oxford |publisher=Oxford University Press |isbn=9780190288716 }} | |||
| ==External links== | ==External links== | ||
| {{Commons category|Ozone layer}} | {{Commons category|Ozone layer}} | ||
| {{Wikisource}} | |||
| * | * | ||
| * http://www.unep.org/ozone/Public_Information/4Aii_PublicInfo_Facts_OzoneLayer.asp | * {{Webarchive|url=https://archive.today/20040702045343/http://www.unep.org/ozone/Public_Information/4Aii_PublicInfo_Facts_OzoneLayer.asp |date=July 2, 2004 }} | ||
| *The delivers maps, datasets and validation reports about the past and current state of the ozone layer. | |||
| * NASA. ''Studying Earth's Environment From Space.'' June 2000. (accessed November 3, 2010) http://www.ccpo.odu.edu/~lizsmith/SEES/index.html. | |||
| {{Earth's atmosphere}} | {{Earth's atmosphere}} | ||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| Bye... :) | |||
Latest revision as of 22:57, 18 October 2024
Region of the stratosphere

The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.
The ozone layer was discovered in 1913 by French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of 5,500–6,000 K (5,230–5,730 °C), except that there was no radiation below a wavelength of about 310 nm at the ultraviolet end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The "Dobson Unit" (DU), a convenient measure of the amount of ozone overhead, is named in his honor.
The ozone layer absorbs 97 to 99 percent of the Sun's medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which otherwise would potentially damage exposed life forms near the surface.
In 1985, atmospheric research revealed that the ozone layer was being depleted by chemicals released by industry, mainly chlorofluorocarbons (CFCs). Concerns that increased UV radiation due to ozone depletion threatened life on Earth, including increased skin cancer in humans and other ecological problems, led to bans on the chemicals, and the latest evidence is that ozone depletion has slowed or stopped. The United Nations General Assembly has designated September 16 as the International Day for the Preservation of the Ozone Layer.
Venus also has a thin ozone layer at an altitude of 100 kilometers above the planet's surface.
Sources
Main article: Ozone–oxygen cycle
The photochemical mechanisms that give rise to the ozone layer were discovered by the British physicist Sydney Chapman in 1930. Ozone in the Earth's stratosphere is created by ultraviolet light striking ordinary oxygen molecules containing two oxygen atoms (O2), splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O2 to create ozone, O3. The ozone molecule is unstable (although, in the stratosphere, long-lived) and when ultraviolet light hits ozone it splits into a molecule of O2 and an individual atom of oxygen, a continuing process called the ozone–oxygen cycle. Chemically, this can be described as:
About 90 percent of the ozone in the atmosphere is contained in the stratosphere. Ozone concentrations are greatest between about 20 and 40 kilometres (66,000 and 131,000 ft), where they range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of the air at sea level, it would be only 3 millimetres (1⁄8 inch) thick.
Ultraviolet light

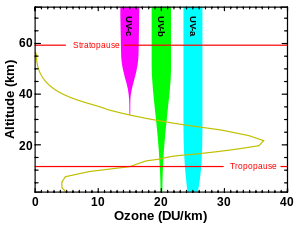
Although the concentration of the ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation coming from the Sun. Extremely short or vacuum UV (10–100 nm) is screened out by nitrogen. UV radiation capable of penetrating nitrogen is divided into three categories, based on its wavelength; these are referred to as UV-A (400–315 nm), UV-B (315–280 nm), and UV-C (280–100 nm).
UV-C, which is very harmful to all living things, is entirely screened out by a combination of dioxygen (< 200 nm) and ozone (> about 200 nm) by around 35 kilometres (115,000 ft) altitude. UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause cataracts, immune system suppression, and genetic damage, resulting in problems such as skin cancer. The ozone layer (which absorbs from about 200 nm to 310 nm with a maximal absorption at about 250 nm) is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at the top of the atmosphere is 350 million times stronger than at the Earth's surface. Nevertheless, some UV-B, particularly at its longest wavelengths, reaches the surface, and is important for the skin's production of vitamin D in mammals.
Ozone is transparent to most UV-A, so most of this longer-wavelength UV radiation reaches the surface, and it constitutes most of the UV reaching the Earth. This type of UV radiation is significantly less harmful to DNA, although it may still potentially cause physical damage, premature aging of the skin, indirect genetic damage, and skin cancer.
Distribution in the stratosphere
| This section needs additional citations for verification. Please help improve this article by adding citations to reliable sources in this section. Unsourced material may be challenged and removed. (February 2013) (Learn how and when to remove this message) |

The thickness of the ozone layer varies worldwide and is generally thinner near the equator and thicker near the poles. Thickness refers to how much ozone is in a column over a given area and varies from season to season. The reasons for these variations are due to atmospheric circulation patterns and solar intensity.
The majority of ozone is produced over the tropics and is transported towards the poles by stratospheric wind patterns. In the northern hemisphere these patterns, known as the Brewer–Dobson circulation, make the ozone layer thickest in the spring and thinnest in the fall. When ozone is produced by solar UV radiation in the tropics, it is done so by circulation lifting ozone-poor air out of the troposphere and into the stratosphere where the sun photolyzes oxygen molecules and turns them into ozone. Then, the ozone-rich air is carried to higher latitudes and drops into lower layers of the atmosphere.
Research has found that the ozone levels in the United States are highest in the spring months of April and May and lowest in October. While the total amount of ozone increases moving from the tropics to higher latitudes, the concentrations are greater in high northern latitudes than in high southern latitudes, with spring ozone columns in high northern latitudes occasionally exceeding 600 DU and averaging 450 DU whereas 400 DU constituted a usual maximum in the Antarctic before anthropogenic ozone depletion. This difference occurred naturally because of the weaker polar vortex and stronger Brewer–Dobson circulation in the northern hemisphere owing to that hemisphere's large mountain ranges and greater contrasts between land and ocean temperatures. The difference between high northern and southern latitudes has increased since the 1970s due to the ozone hole phenomenon. The highest amounts of ozone are found over the Arctic during the spring months of March and April, but the Antarctic has the lowest amounts of ozone during the summer months of September and October,

Depletion
Main article: Ozone depletion
The ozone layer can be depleted by free radical catalysts, including nitric oxide (NO), nitrous oxide (N2O), hydroxyl (OH), atomic chlorine (Cl), and atomic bromine (Br). While there are natural sources for all of these species, the concentrations of chlorine and bromine increased markedly in recent decades because of the release of large quantities of man-made organohalogen compounds, especially chlorofluorocarbons (CFCs) and bromofluorocarbons. These highly stable compounds are capable of surviving the rise to the stratosphere, where Cl and Br radicals are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 100,000 ozone molecules. By 2009, nitrous oxide was the largest ozone-depleting substance (ODS) emitted through human activities.
The breakdown of ozone in the stratosphere results in reduced absorption of ultraviolet radiation. Consequently, unabsorbed and dangerous ultraviolet radiation is able to reach the Earth's surface at a higher intensity. Ozone levels have dropped by a worldwide average of about 4 percent since the late 1970s. For approximately 5 percent of the Earth's surface, around the north and south poles, much larger seasonal declines have been seen, and are described as "ozone holes". "Ozone holes" are actually patches in the ozone layer in which the ozone is thinner. The thinnest parts of the ozone are at the polar points of Earth's axis. The discovery of the annual depletion of ozone above the Antarctic was first announced by Joe Farman, Brian Gardiner and Jonathan Shanklin, in a paper which appeared in Nature on May 16, 1985.
Regulation attempts have included but not have been limited to the Clean Air Act implemented by the United States Environmental Protection Agency. The Clean Air Act introduced the requirement of National Ambient Air Quality Standards (NAAQS) with ozone pollutions being one of six criteria pollutants. This regulation has proven to be effective since counties, cities and tribal regions must abide by these standards and the EPA also provides assistance for each region to regulate contaminants. Effective presentation of information has also proven to be important in order to educate the general population of the existence and regulation of ozone depletion and contaminants. A scientific paper was written by Sheldon Ungar in which the author explores and studies how information about the depletion of the ozone, climate change and various related topics. The ozone case was communicated to lay persons "with easy-to-understand bridging metaphors derived from the popular culture" and related to "immediate risks with everyday relevance". The specific metaphors used in the discussion (ozone shield, ozone hole) proved quite useful and, compared to global climate change, the ozone case was much more seen as a "hot issue" and imminent risk. Lay people were cautious about a depletion of the ozone layer and the risks of skin cancer.
Satellites burning up upon re-entry into Earth's atmosphere produce aluminum oxide (Al2O3) nanoparticles that endure in the atmosphere for decades. Estimates for 2022 alone were ~17 metric tons (~30 kg of nanoparticles per ~250 kg satellite). Increasing populations of satellite constellations can eventually lead to significant ozone depletion.
"Bad" ozone can cause adverse health risks respiratory effects (difficulty breathing) and is proven to be an aggravator of respiratory illnesses such as asthma, COPD and emphysema. That is why many countries have set in place regulations to improve "good" ozone and prevent the increase of "bad" ozone in urban or residential areas. In terms of ozone protection (the preservation of "good" ozone) the European Union has strict guidelines on what products are allowed to be bought, distributed or used in specific areas. With effective regulation, the ozone is expected to heal over time.

In 1978, the United States, Canada and Norway enacted bans on CFC-containing aerosol sprays that damage the ozone layer but the European Community rejected a similar proposal. In the U.S., chlorofluorocarbons continued to be used in other applications, such as refrigeration and industrial cleaning, until after the discovery of the Antarctic ozone hole in 1985. After negotiation of an international treaty (the Montreal Protocol), CFC production was capped at 1986 levels with commitments to long-term reductions. This allowed for a ten-year phase-in for developing countries (identified in Article 5 of the protocol). Since then, the treaty was amended to ban CFC production after 1995 in developed countries, and later in developing countries. All of the world's 197 countries have signed the treaty. Beginning January 1, 1996, only recycled or stockpiled CFCs were available for use in developed countries like the US. The production phaseout was possible because of efforts to ensure that there would be substitute chemicals and technologies for all ODS uses.
On August 2, 2003, scientists announced that the global depletion of the ozone layer might be slowing because of the international regulation of ozone-depleting substances. In a study organized by the American Geophysical Union, three satellites and three ground stations confirmed that the upper-atmosphere ozone-depletion rate slowed significantly over the previous decade. Some breakdown was expected to continue because of ODSs used by nations which have not banned them, and because of gases already in the stratosphere. Some ODSs, including CFCs, have very long atmospheric lifetimes ranging from 50 to over 100 years. It has been estimated that the ozone layer will recover to 1980 levels near the middle of the 21st century. A gradual trend toward "healing" was reported in 2016.
Compounds containing C–H bonds (such as hydrochlorofluorocarbons, or HCFCs) have been designed to replace CFCs in certain applications. These replacement compounds are more reactive and less likely to survive long enough in the atmosphere to reach the stratosphere where they could affect the ozone layer. While being less damaging than CFCs, HCFCs can have a negative impact on the ozone layer, so they are also being phased out. These in turn are being replaced by hydrofluorocarbons (HFCs) and other compounds that do not destroy stratospheric ozone at all.
The residual effects of CFCs accumulating within the atmosphere lead to a concentration gradient between the atmosphere and the ocean. This organohalogen compound is able to dissolve into the ocean's surface waters and is able to act as a time-dependent tracer. This tracer helps scientists study ocean circulation by tracing biological, physical and chemical pathways.
Implications for astronomy
As ozone in the atmosphere prevents most energetic ultraviolet radiation reaching the surface of the Earth, astronomical data in these wavelengths have to be gathered from satellites orbiting above the atmosphere and ozone layer. Most of the light from young hot stars is in the ultraviolet and so study of these wavelengths is important for studying the origins of galaxies. The Galaxy Evolution Explorer, GALEX, is an orbiting ultraviolet space telescope launched on April 28, 2003, which operated until early 2012.
-
 This GALEX image of the Cygnus Loop nebula could not have been taken from the surface of the Earth because the ozone layer blocks the ultra-violet radiation emitted by the nebula.
This GALEX image of the Cygnus Loop nebula could not have been taken from the surface of the Earth because the ozone layer blocks the ultra-violet radiation emitted by the nebula.
See also
- Cambrian explosion
- Nuclear winter
- Oxygen
- United Nations Environment Programme
- Short-lived climate pollutants
References
- "Ozone Basics". NOAA. March 20, 2008. Archived from the original on November 21, 2017. Retrieved January 29, 2007.
- McElroy, C.T.; Fogal, P.F. (2008). "Ozone: From discovery to protection". Atmosphere-Ocean. 46 (1): 1–13. Bibcode:2008AtO....46....1M. doi:10.3137/ao.460101. S2CID 128994884.
- "Ozone layer". Archived from the original on May 2, 2021. Retrieved September 23, 2007.
- An Interview with Lee Thomas, EPA's 6th Administrator. Video, Transcript (see p13). April 19, 2012.
- SPACE.com staff (October 11, 2011). "Scientists discover Ozone Layer on Venus". SPACE.com. Purch. Retrieved October 3, 2015.
- "NASA Facts Archive". Archived from the original on April 6, 2013. Retrieved June 9, 2011.
- Matsumi, Y.; Kawasaki, M. (2003). "Photolysis of Atmospheric Ozone in the Ultraviolet Region" (PDF). Chem. Rev. 103 (12): 4767–4781. doi:10.1021/cr0205255. PMID 14664632. Archived from the original (PDF) on June 17, 2012. Retrieved March 14, 2015.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. (2010). "Review: Ultraviolet radiation and skin cancer". International Journal of Dermatology. 49 (9): 978–986. doi:10.1111/j.1365-4632.2010.04474.x. PMID 20883261. S2CID 22224492.
- ^ Tabin, Shagoon (2008). Global Warming: The Effect Of Ozone Depletion. APH Publishing. p. 194. ISBN 9788131303962. Retrieved January 12, 2016.
- "Nasa Ozone Watch: Ozone facts". ozonewatch.gsfc.nasa.gov. Retrieved September 16, 2021.
- Douglass, Anne R.; Newman, Paul A.; Solomon, Susan (2014). "The Antarctic ozone hole: An update". Physics Today. 67 (7). American Institute of Physics: 42–48. Bibcode:2014PhT....67g..42D. doi:10.1063/PT.3.2449. hdl:1721.1/99159.
- "Halocarbons and Other Gases". Emissions of Greenhouse Gases in the United States 1996. Energy Information Administration. 1997. Archived from the original on June 29, 2008. Retrieved June 24, 2008.
- "NOAA Study Shows Nitrous Oxide Now Top Ozone-Depleting Emission". NOAA. August 27, 2009. Retrieved November 8, 2011.
- "ozone layer | National Geographic Society". education.nationalgeographic.org. Retrieved May 30, 2022.
- US EPA, OAR (December 14, 2016). "Ozone Implementation Regulatory Actions". www.epa.gov. Retrieved May 30, 2022.
- Ungar, Sheldon (July 2000). "Knowledge, ignorance and the popular culture: climate change versus the ozone hole". Public Understanding of Science. 9 (3): 297–312. doi:10.1088/0963-6625/9/3/306. ISSN 0963-6625. S2CID 7089937.
- ^ Ferreira, Jose P.; Huang, Ziyu; Nomura, Ken-ichi; Wang, Joseph (June 11, 2024). "Potential Ozone Depletion From Satellite Demise During Atmospheric Reentry in the Era of Mega-Constellations". Geophysical Research Letters. doi:10.1029/2024GL109280.
- Zhang, Junfeng (Jim); Wei, Yongjie; Fang, Zhangfu (2019). "Ozone Pollution: A Major Health Hazard Worldwide". Frontiers in Immunology. 10: 2518. doi:10.3389/fimmu.2019.02518. ISSN 1664-3224. PMC 6834528. PMID 31736954.
- "Ozone Regulation". ec.europa.eu. Retrieved May 30, 2022.
- US EPA, OAR (July 15, 2015). "International Treaties and Cooperation about the Protection of the Stratospheric Ozone Layer". www.epa.gov. Retrieved May 30, 2022.
- Morrisette, Peter M. (1989). "The Evolution of Policy Responses to Stratospheric Ozone Depletion". Natural Resources Journal. 29: 793–820. Retrieved April 20, 2010.
- An Interview with Lee Thomas, EPA's 6th Administrator. Video, Transcript (see p15). April 19, 2012.
- "Amendments to the Montreal Protocol". EPA. August 19, 2010. Retrieved March 28, 2011.
- "Brief Questions and Answers on Ozone Depletion". EPA. June 28, 2006. Retrieved November 8, 2011.
- "Stratospheric Ozone and Surface Ultraviolet Radiation" (PDF). Scientific Assessment of Ozone Depletion: 2010. WMO. 2011. Retrieved March 14, 2015.
- Solomon, Susan, et al. (June 30, 2016). "Emergence of healing in the Antarctic ozone layer". Science. 353 (6296): 269–74. Bibcode:2016Sci...353..269S. doi:10.1126/science.aae0061. hdl:1721.1/107197. PMID 27365314.
- "Ozone Depletion Glossary". EPA. Retrieved September 3, 2008.
- Fine, Rana A. (2011). "Observations of CFCs and SF6 as Ocean Tracers" (PDF). Annual Review of Marine Science. 3: 173–95. Bibcode:2011ARMS....3..173F. doi:10.1146/annurev.marine.010908.163933. PMID 21329203. Archived from the original (PDF) on February 10, 2015.
- "ozone layer". National Geographic Society. May 9, 2011. Retrieved September 16, 2021.
Further reading
- Science
- Andersen, S. O. (2015). "Lessons from the stratospheric ozone layer protection for climate". Journal of Environmental Studies and Sciences. 5 (2): 143–162. Bibcode:2015JEnSS...5..143A. doi:10.1007/s13412-014-0213-9. S2CID 129725437.
- Andersen, S.O.; Sarma, K.M.; Sinclair, L. (2012). Protecting the Ozone Layer: The United Nations History. Taylor & Francis. ISBN 978-1-84977-226-6.
- Ritchie, Hannah, "What We Learned from Acid Rain: By working together, the nations of the world can solve climate change", Scientific American, vol. 330, no. 1 (January 2024), pp. 75–76. "ountries will act only if they know others are willing to do the same. With acid rain, they did act collectively.... We did something similar to restore Earth's protective ozone layer.... he cost of technology really matters.... In the past decade the price of solar energy has fallen by more than 90 percent and that of wind energy by more than 70 percent. Battery costs have tumbled by 98 percent since 1990, bringing the price of electric cars down with them....he stance of elected officials matters more than their party affiliation.... Change can happen – but not on its own. We need to drive it." (p. 76.)
- United Nations Environment Programme (2010). Environmental Effects of Ozone Depletion and its Interactions with Climate Change: 2010 Assessment. Nairobi: UNEP.
- Velders, G. J. M.; Fahey, D. W.; Daniel, J. S.; McFarland, M.; Andersen, S. O. (2009). "The large contribution of projected HFC emissions to future climate forcing". Proceedings of the National Academy of Sciences. 106 (27): 10949–10954. Bibcode:2009PNAS..10610949V. doi:10.1073/pnas.0902817106. PMC 2700150. PMID 19549868. S2CID 3743609.
- Velders, Guus J.M.; Andersen, Stephen O.; Daniel, John S.; Fahey, David W.; McFarland, Mack (2007). "The Importance of the Montreal Protocol in Protecting Climate". Proceedings of the National Academy of Sciences of the United States of America. 104 (12): 4814–4819. Bibcode:2007PNAS..104.4814V. doi:10.1073/pnas.0610328104. PMC 1817831. PMID 17360370.
- Policy
- Zaelke, Durwood; Borgford-Parnell, Nathan (2015). "The importance of phasing down hydrofluorocarbons and other short-lived climate pollutants". Journal of Environmental Studies and Sciences. 5 (2): 169–175. Bibcode:2015JEnSS...5..169Z. doi:10.1007/s13412-014-0215-7. S2CID 128974741.
- Xu, Y.; Zaelke, D.; Velders, G. J. M.; Ramanathan, V. (2013). "The role of HFCS in mitigating 21st century climate change". Atmospheric Chemistry and Physics. 13 (12): 6083–6089. Bibcode:2013ACP....13.6083X. doi:10.5194/acp-13-6083-2013.
- Molina, M.; Zaelke, D.; Sarma, K. M.; Andersen, S. O.; Ramanathan, V.; Kaniaru, D. (2009). "Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions". Proceedings of the National Academy of Sciences. 106 (49): 20616–20621. doi:10.1073/pnas.0902568106. PMC 2791591. PMID 19822751. S2CID 13240115.
- Anderson, S. O.; Sarma, M. K.; Taddonio, K. (2007). Technology Transfer for the Ozone Layer: Lessons for Climate Change. London: Earthscan. ISBN 9781849772846.
- Benedick, Richard Elliot; World Wildlife Fund (U.S.); Institute for the Study of Diplomacy. Georgetown University. (1998). Ozone Diplomacy: New Directions in Safeguarding the Planet (2nd ed.). Harvard University Press. ISBN 978-0-674-65003-9. (Ambassador Benedick was the Chief U.S. Negotiator at the meetings that resulted in the Montreal Protocol.)
- Chasek, P. S.; Downie, David L.; Brown, J. W. (2013). Global Environmental Politics (6th ed.). Boulder: Westview Press. ISBN 9780813348971.
- Grundmann, Reiner (2001). Transnational Environmental Policy: Reconstructing Ozone. Psychology Press. ISBN 978-0-415-22423-9.
- Parson, E. (2003). Protecting the Ozone Layer: Science and Strategy. Oxford: Oxford University Press. ISBN 9780190288716.
External links
- Stratospheric ozone: an electronic textbook
- Ozone Layer Info Archived July 2, 2004, at archive.today
- The CAMS stratospheric ozone service delivers maps, datasets and validation reports about the past and current state of the ozone layer.
| Earth's atmosphere | |
|---|---|

