| Revision as of 21:48, 10 April 2006 view sourceLigulem (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers28,245 edits →Notes and references: Could we please have normal font size for the references? There is no need to have such an illegible small font. This is not a paper encyclopedia.← Previous edit | Revision as of 22:37, 10 April 2006 view source Eternal Equinox (talk | contribs)4,840 edits Rv, many featured articles have smaller references so that there is no violation of Misplaced Pages:Article size.Next edit → | ||
| Line 1,166: | Line 1,166: | ||
| ==Notes and references== | ==Notes and references== | ||
| <div style="font-size:85%"> | |||
| <references/> | <references/> | ||
| </div> | |||
| ==External links== | ==External links== | ||
Revision as of 22:37, 10 April 2006

Acquired immunodeficiency syndrome, or Acquired Immune Deficiency Syndrome (AIDS), is a collection of symptoms and infections resulting from the specific damage to the immune system caused by infection with the human immunodeficiency virus (HIV), the late stage of which leaves individuals prone to opportunistic infections and tumours. Although treatments for both AIDS and HIV exist to slow the virus' progression, there is no known cure.
HIV is transmitted through direct contact of a mucous membrane or the bloodstream with a bodily fluid containing HIV, such as blood, semen, vaginal fluid, preseminal fluid or breast milk. This transmission can come in the form of: penetrative (anal or vaginal) sex; oral sex; blood transfusion; contaminated needles; exchange between mother and infant during pregnancy, childbirth, or breastfeeding; or other exposure to one of the above bodily fluids.
Most researchers believe that HIV originated in sub-Saharan Africa during the twentieth century; it is now a pandemic, with more than 40 million people now living with the disease worldwide. As of January 2006, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) estimate that AIDS has killed more than 25 million people since it was first recognized on December 1, 1981, making it one of the most destructive pandemics in recorded history. In 2005 alone, AIDS claimed between an estimated 2.8 and 3.6 million, of which more than 570,000 were children. Many of these deaths are occurring in sub-Saharan Africa, retarding economic growth by destroying human capital. Antiretroviral treatment reduces both the mortality and the morbidity of HIV infection, but routine access to antiretroviral medication is not available in all countries. HIV/AIDS stigma is more severe than that associated with other life-threatening conditions and extends beyond the disease itself to providers and even volunteers involved with the care of people with HIV.
Medical condition| HIV/AIDS | |
|---|---|
| Specialty | Infectious diseases |
Infection by HIV
Further information: HIV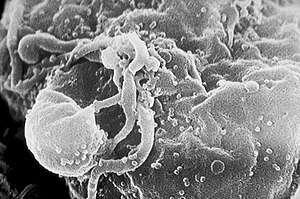
AIDS is the most severe manifestation of infection with HIV. HIV is a retrovirus that primarily infects vital components of the human immune system such as CD4+ T cells, macrophages and dendritic cells. It directly and indirectly destroys CD4+ T cells. As CD4+ T cells are required for the proper functioning of the immune system, when HIV kills CD4+ T cells so that there are less than 200 CD4+ T cells per µl blood, cellular immunity is lost, leading to AIDS. Acute HIV infection progresses over time to clinical latent HIV infection and then to early symptomatic HIV infection and later, to AIDS, which is identified on the basis of the amount of CD4+ T cells in the blood and the presence of certain infections.
In the absence of antiretroviral therapy, the median time of progression from HIV infection to AIDS is nine to ten years, and the median survival time after developing AIDS is only 9.2 months. However, the rate of clinical disease progression varies widely between individuals, from two weeks up to 20 years. Many factors affect the rate of progression. These include factors that influence the body's ability to defend against HIV such as the infected person's general immune function. Older people have weaker immune systems so are at more of a risk of faster disease progression than younger people. Poor access to health care and the existence of coexisting infections such as tuberculosis may predispose to faster disease progression. The infected person's genetic inheritance plays an important role and some people are resistant to certain strains of HIV. An example of this is people with the CCR5-Δ32 mutation; these people are resistant to infection with certain strains of HIV. The strain of HIV that infects someone plays a role in the disease progression rate. HIV is genetically variable and exists as different strains which cause different rates of clinical disease progression. The use of highly active antiretroviral therapy prolongs both the median time of progression to AIDS and the median survival time.
Diagnosis
Since June 18, 1981, many definitions have been developed for epidemiological surveillance such as the Bangui definition and the 1994 expanded World Health Organization AIDS case definition. However, clinical staging of patients was not an intended use for these systems as they are neither sensitive nor specific. In developing countries, the World Health Organizations (WHO) staging system for HIV infection and disease, using clinical and laboratory data, is used and in developed countries, the Centers for Disease Control (CDC) Classification System is used.
WHO Disease Staging System for HIV Infection and Disease
Main article: WHO Disease Staging System for HIV Infection and DiseaseIn 1990, the World Health Organization (WHO) grouped these infections and conditions together by introducing a staging system for patients infected with HIV-1. An update took place in September 2005. Most of these conditions are opportunistic infections that can be easily treated in healthy people.
- Stage I: HIV disease is asymptomatic and not categorized as AIDS
- Stage II: include minor mucocutaneous manifestations and recurrent upper respiratory tract infections
- Stage III: includes unexplained chronic diarrhoea for longer than a month, severe bacterial infections and pulmonary tuberculosis
- Stage IV includes toxoplasmosis of the brain, candidiasis of the oesophagus, trachea, bronchi or lungs and Kaposi's sarcoma; these diseases are indicators of AIDS.
CDC Classification System for HIV Infection
Main article: CDC Classification System for HIV InfectionThe Centers for Disease Control and Prevention (CDC) has a slightly different definition of AIDS. In 1993, the CDC expanded their definition of AIDS to include healthy HIV positive people with a CD4 positive T cell count of less than 200 per µl of blood. The majority of new AIDS cases in developed countries use this definition.
HIV test
Main article: HIV testApproximately half of those infected with HIV do not know their HIV status until their AIDS diagnosis with a HIV test. The screening of donor blood and blood products used in medicine and medical research is by this test. Typical HIV tests, including the HIV enzyme immunoassay and the Western blot assay, detect HIV antibodies in serum, plasma, oral fluid, dried blood spot or urine of patients. Commercially available tests to detect other HIV antigens, HIV-RNA, and HIV-DNA in order to detect HIV infection prior to the development of detectable antibodies are available. However, for the diagnosis of HIV infection these assays are not specifically approved, but are nonetheless routinely used in developed countries.
Symptoms and Complications

The symptoms of AIDS are primarily the result of conditions that do not normally develop in individuals with healthy immune systems. Most of these conditions are infections caused by bacteria, viruses, fungi and parasites that are normally controlled by the elements of the immune system that HIV damages. Opportunistic infections are common in people with AIDS. HIV affects nearly every organ system. People with AIDS also have an increased risk of developing various cancers such as Kaposi sarcoma, cervical cancer and cancers of the immune system known as lymphomas.
Additionally, people with AIDS often have systemic symptoms of infection like fevers, sweats (particularly at night), swollen glands, chills, weakness, and weight loss. After the diagnosis of AIDS is made, the current average survival time with antiretroviral therapy is estimated to be now more than 5 years, but because new treatments continue to be developed and because HIV continues to evolve resistance to treatments, estimates of survival time are likely to continue to change. Without antiretroviral therapy, death normally occurs within a year. Most patients die from opportunistic infections or malignancies associated with the progressive failure of the immune system.
The rate of clinical disease progression varies widely between individuals and has been shown to be affected by many factors such as host susceptibility and immune function health care and co-infections, as well as factors relating to the viral strain. The specific opportunistic infections that AIDS patients develop depend in part on the prevalence of these infections in the geographic area in which the patient lives.
The major pulmonary illnesses
- Pneumocystis jiroveci pneumonia (originally known as Pneumocystis carinii pneumonia, often-abbreviated PCP) is relatively rare in normal, immunocompetent people but common among HIV-infected individuals. Before the advent of effective diagnosis, treatment and routine prophylaxis in Western countries, it was a common immediate cause of death. In developing countries, it is still one of the first indications of AIDS in untested individuals, although it does not generally occur unless the CD4 count is less than 200 per µl.
- Tuberculosis (TB) is unique among infections associated with HIV in that it is transmissible to immunocompetent persons via the respiratory route, is easily treatable once identified, may occur in early-stage HIV disease, and is preventable with drug therapy. However, multi-drug resistance is a potentially serious problem. Even though its incidence has declined because of the use of directly observed therapy and other improved practices in Western countries, this is not the case in developing countries where HIV is most prevalent. In early-stage HIV infection (CD4 count >300 cells per µl), TB typically presents as a pulmonary disease. In advanced HIV infection, TB may present atypically and extrapulmonary TB is common infecting bone marrow, bone, urinary and gastrointestinal tracts, liver, regional lymph nodes, and the central nervous system.
The major gastro-intestinal illnesses
- Oesophagitis is an inflammation of the lining of the lower end of the oesophagus (gullet or swallowing tube leading to the stomach). In HIV infected individuals, this is normally due to fungal (candidiasis) or viral (herpes simplex-1 or cytomegalovirus) infections. In rare cases, it could be due to mycobacteria.
- Unexplained chronic diarrhoea in HIV infection is due to many possible causes, including common bacterial (Salmonella, Shigella, Listeria, Campylobacter, or Escherichia coli) and parasitic infections, and uncommon opportunistic infections such as cryptosporidiosis, microsporidiosis, Mycobacterium avium complex (MAC) and cytomegalovirus (CMV) colitis. In some cases, diarrhoea may be a side effect of several drugs used to treat HIV, or it may simply accompany HIV infection, particularly during primary HIV infection. It may also be a side effect of antibiotics used to treat bacterial causes of diarrhoea (common for Clostridium difficile). In the later stages of HIV infection, diarrhoea is thought to be a reflection of changes in the way the intestinal tract absorbs nutrients, and may be an important component of HIV-related wasting.
The major neurological illnesses
- Toxoplasmosis is a disease caused by the single-celled parasite called Toxoplasma gondii. T. gondii usually infects the brain causing toxoplasma encephalitis and it can infect and cause disease in the eyes and lungs.
- Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease, in which the gradual destruction of the myelin sheath covering the axons of nerve cells impairs the transmission of nerve impulses. It is caused by a virus called JC virus which occurs in 70% of the population in latent form, causing disease only when the immune system has been severely weakened, as is the case for AIDS patients. It progresses rapidly, usually causing death within months of diagnosis.
- AIDS dementia complex (ADC) is a metabolic encephalopathy induced by HIV infection and fuelled by immune activation of HIV infected brain macrophages and microglia which secrete neurotoxins of both host and viral origin. Specific neurological impairments are manifested by cognitive, behavioural, and motor abnormalities that occur after years of HIV infection and is associated with low CD4+ T cell levels and high plasma viral loads. Prevalence is 10-20% in Western countries but only 1-2% of HIV infections in India. This difference is possibly due to the HIV subtype in India.
- Cryptococcal meningitis is an infection of the meninges (the membrane covering the brain and spinal cord) by the fungus Cryptococcus neoformans. It can cause fevers, headache, fatigue, nausea, and vomiting. Patients may also develop seizures and confusion. If untreated, it can be lethal.
The major HIV-associated malignancies
Patients with HIV infection have substantially increased incidence of several malignancies. Several of these, Kaposi's sarcoma, high-grade lymphoma, and cervical cancer confer a diagnosis of AIDS when they occur in an HIV-infected person.
- Kaposi's sarcoma (KS) is the most common tumour in HIV-infected patients. A gammaherpesvirus called Kaposi's sarcoma-associated herpes virus (KSHV) causes it. The appearance of this tumour in young gay men in 1981 was one of the first signals of the AIDS epidemic. It often appears as purplish nodules on the skin, but can affect other organs, especially the mouth, gastrointestinal tract, and lungs.
- High-grade B cell lymphomas such as Burkitt's lymphoma, Burkitt's-like lymphoma, and diffuse large B-cell lymphoma (DLBCL), including primary central nervous system lymphoma, have substantially increased incidence in HIV-infected patients and often portend a poor prognosis. In some cases these lymphomas are AIDS-defining. Primary effusion lymphoma is less common. Epstein-Barr virus (EBV) or KSHV cause many of these lymphomas.
- Cervical cancer in HIV-infected women is considered AIDS-defining. It is caused by human papillomavirus (HPV).
- In addition to the AIDS-defining tumours listed above, HIV-infected patients are at increased risk of certain other tumours, such as Hodgkin's disease and anal and rectal carcinomas. However, the incidence of many common tumours, such as breast cancer or colon cancer, does not increase in HIV-infected patients. Co-infection of patients with an oncogenic DNA virus, especially Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV), and human papillomavirus (HPV) is the root cause of most AIDS-associated malignancies. In areas where HAART is extensively used to treat AIDS, the incidence of many AIDS-related malignancies has decreased, but at the same time malignancies overall have become the most common cause of death of HIV-infected patients.
Other opportunistic infections
Patients with AIDS and severe immunosuppression often develop opportunistic infections that present with non-specific symptoms, especially low-grade fevers and weight loss. These include infection with Mycobacterium avium-intracellulare and cytomegalovirus (CMV). CMV can cause colitis, as described above, and CMV retinitis can cause blindness. Penicilliosis due to Penicillium marneffei is now the third most common opportunistic infection (after extrapulmonary tuberculosis and cryptococcosis) in HIV-positive individuals within the endemic area of Southeast Asia.
Transmission and prevention
| Estimated per act risk for acquisition of HIV by exposure route | ||||
|---|---|---|---|---|
| Exposure Route | Estimated infections per 10,000 exposures to an infected source | |||
| Blood Transfusion | 9,000 | |||
| Childbirth | 2,500 | |||
| Needle-sharing injection drug use | 67 | |||
| Receptive anal intercourse | 50 | |||
| Percutaneous needle stick | 30 | |||
| Receptive penile-vaginal intercourse | 10 | |||
| Insertive anal intercourse | 6.5 | |||
| Insertive penile-vaginal intercourse | 5 | |||
| Receptive oral intercourse | 1 | |||
| Insertive oral intercourse | 0.5 | |||
| assuming no condom use Source refers to oral intercourse performed on a man | ||||
The three main transmission routes of HIV are sexual contact, exposure to infected body fluids or tissues and from mother to foetus or child during perinatal period. It is possible to find HIV in the saliva, tears and urine of infected individuals, but due to the low concentration of virus in these biological liquids, the risk is negligible.
Sexual contact
The majority of HIV infections are acquired through unprotected sexual relations between HIV positive disconcordant couples. Sexual transmission occurs with the contact between sexual secretions of one partner with the rectal, genital or mouth mucous membranes of another. Unprotected receptive sexual acts are riskier than unprotected insertive sexual acts, with the risk for transmitting HIV from an infected partner to an uninfected partner through unprotected insertive anal intercourse greater than the risk for transmission through vaginal intercourse or oral sex. Oral sex is not without its risks as HIV is transmissible through both insertive and receptive oral sex.
Sexually transmitted infections (STI) increase the risk of HIV transmission and infection because they cause the disruption of the normal epithelial barrier by genital ulceration and/or microulceration; and by accumulation of pools of HIV-susceptible or HIV-infected cells (lymphocytes and macrophages) in semen and vaginal secretions. Epidemiological studies from sub-Saharan Africa, Europe and North America have suggested that there is approximately a four times greater risk of becoming HIV-infected in the presence of a genital ulcer such as caused by syphilis and/or chancroid; and a significant though lesser increased risk in the presence of STIs such as gonorrhoea, chlamydial infection and trichomoniasis which cause local accumulations of lymphocytes and macrophages.
Transmission of HIV depends on the infectiousness of the index case and the susceptibility of the uninfected partner. Infectivity seems to vary during the course of illness and is not constant between individuals. An undetectable plasma viral load does not necessarily indicate a low viral load in the seminal liquid or genital secretions. Each 10-fold increment of seminal HIV RNA is associated with an 81% increased rate of HIV transmission. Women are more susceptible to HIV-1 infection due to hormonal changes, vaginal microbial ecology and physiology, and a higher prevalence of sexually transmitted diseases. People who are infected with HIV can still be infected by other, more virulent strains.
During a sexual act, only condoms, be they male or female, can reduce the chances of infection with HIV and other STIs and the chances of becoming pregnant. They must be used during all penetrative sexual intercourse with a partner who is HIV positive or whose status is unknown. The effective use of condoms and screening of blood transfusion in North America, Western and Central Europe is credited with the low rates of AIDS in these regions.
Promoting condom use, however, has often proved controversial and difficult. Many religious groups, most visibly the Roman Catholic Church, have opposed the use of condoms on religious grounds, and have sometimes seen condom promotion as an affront to the promotion of marriage, monogamy and sexual morality. Other religious groups, such as the Scottish Episcopalians, have argued that preventing HIV infection is a moral task in itself and that condoms are therefore acceptable or even praiseworthy from a religious point of view.
The male latex condom, if used correctly without oil-based lubricants, is the single most efficient available technology to reduce the sexual transmission of HIV and other sexually transmitted infections. Lubricants containing oil, such as petroleum jelly, or butter, weaken latex condoms making them porous. If necessary, water-based lubricants are recommended. However, it is not recommended to use a lubricant for fellatio. Latex degrades over time, making them porous, which is why condoms have expiration dates. In Europe and the United States, condoms have to conform to European (EC 600) or American (D3492) standards to be considered protective against HIV transmission.
The female condom is an alternative to the male condom and is made from polyurethane, which allows it to be used in the presence of oil-based lubricants. They are larger than male condoms and have a stiffened ring-shaped opening, and are designed to be inserted into the vagina. The female condom contains an inner ring which keeps the condom in place inside the vagina—inserting the female condom requires squeezing this ring.
With consistent and correct use of condoms, there is a very low risk of HIV infection. Studies on couples where one partner is infected show that with consistent condom use, HIV infection rates for the uninfected partner are below 1% per year.
The United States government and health organizations both endorse the ABC Approach to lower the risk of acquiring AIDS during sex:
- Abstinence or delay of sexual activity, especially for youth,
- Being faithful, especially for those in committed relationships,
- Condom use, for those who engage in risky behaviour.
This approach has been very successful in Uganda, where HIV prevalence has decreased from 15% to 5%. However, more has been done than implementing the ABC Approach—"Uganda has pioneered approaches towards reducing stigma, bringing discussion of sexual behaviour out into the open, involving HIV-infected people in public education, persuading individuals and couples to be tested and counseled, improving the status of women, involving religious organizations, enlisting traditional healers, and much more." (Edward Green, Harvard medical anthropologist). There is no conclusive proof that abstinence-only programs have been successful in any country in the world in changing sexual behaviour or in reducing HIV transmission. This is why condom use is heavily co-promoted. There is considerable overlap with the CNN Approach. This is:
- Condom use, for those who engage in risky behaviour.
- Needles, use clean ones
- Negotiating skills; negotiating safer sex with a partner and empowering women to make smart choices
Criticism of the ABC approach is widespread because a faithful partner of an unfaithful partner is at risk of contracting HIV. Many think that the combination of the CNN approach with the ABC approach will be the optimum prevention platform.
Current research is clarifying the relationship between male circumcision and HIV in differing social and cultural contexts. UNAIDS believes that it is premature to recommend male circumcision services as part of HIV prevention programmes. Moreover, South African medical experts are concerned that the repeated use of unsterilised blades in the ritual circumcision of adolescent boys may be spreading HIV.
Exposure to infected body fluids
This transmission route is particularly important for intravenous drug users, haemophiliacs and recipients of blood transfusions and blood products. Sharing and reusing syringes contaminated with HIV-infected blood represents a major risk for infection with not only HIV but also hepatitis B and hepatitis C. Needle sharing is the cause of one third of all new HIV-infections and 50% of hepatitis C infections in Northern America, China and Eastern Europe. The risk of being infected with HIV from a single prick with a needle that has been used on an HIV infected person though is thought to be about 1 in 150 (see table above). Post-exposure prophylaxis with anti-HIV drugs can further reduce that small risk. Health care workers (nurses, laboratory workers, doctors etc) are also concerned, although more rarely. This route can affect people who give and receive tattoos and piercings. Universal precautions are frequently not followed in both sub-Saharan Africa and much of Asia because of both a shortage of supplies and inadequate training. The WHO estimates that approximately 2.5% of all HIV infections in sub-Saharan Africa are transmitted through unsafe healthcare injections. Because of this, the United Nations General Assembly, supported by universal medical opinion on the matter, has urged the nations of the world to implement universal precautions to prevent HIV transmission in health care settings.
The risk of transmitting HIV to blood transfusion recipients is extremely low in developed countries where improved donor selection and HIV screening is performed. However, according to the WHO, the overwhelming majority of the world's population does not have access to safe blood and "between 5% and 10% of HIV infections worldwide are transmitted through the transfusion of infected blood and blood products".
Medical workers who follow universal precautions or body substance isolation such as wearing latex gloves when giving injections and washing the hands frequently can help prevent infection of HIV.
All AIDS-prevention organizations advise drug-users not to share needles and other material required to prepare and take drugs (including syringes, cotton balls, the spoons, water for diluting the drug, straws, crack pipes etc). It is important that people use new or properly sterilized needles for each injection. Information on cleaning needles using bleach is available from health care and addiction professionals and from needle exchanges. In some developed countries, clean needles are available free in some cities, at needle exchanges or safe injection sites. Additionally, many nations have decriminalized needle possession and made it possible to buy injection equipment from pharmacists without a prescription.
Mother to Child Transmission (MTCT)
The transmission of the virus from the mother to the child can occur in utero during the last weeks of pregnancy and at childbirth. In the absence of treatment, the transmission rate between the mother to the child during pregnancy, labour and delivery is 25%. However, when the mother has access to antiretroviral therapy and gives birth by caesarean section, the rate of transmission is just 1%. A number of factors influence the risk of infection, particularly the viral load of the mother at birth (the higher the load, the higher the risk). Breastfeeding increases the risk of transmission by 10–15%. This risk depends on clinical factors and may vary according to the pattern and duration of breast-feeding.
Studies have shown that antiretroviral drugs, caesarean delivery and formula feeding reduce the chance of transmission of HIV from mother to child. When replacement feeding is acceptable, feasible, affordable, sustainable and safe, HIV-infected mothers are recommended to avoid breast-feeding their infant. Otherwise, exclusive breast-feeding is recommended during the first months of life and should be discontinued as soon as possible. In 2005, around 700,000 children under 15 contracted HIV, mainly through MTCT, with 630,000 of these infections occuring in Africa.
Prevention strategies are well known in developed countries, however, recent epidemiological and behavioural studies in Europe and North America have suggested that a substantial minority of young people continue to engage in high-risk practices and that despite HIV/AIDS knowledge, young people underestimate their own risk of becoming infected with HIV. However, transmission of HIV between intravenous drug users has clearly decreased, and HIV transmission by blood transfusion has become quite rare in developed countries.
Treatment
There is currently no cure or vaccine against HIV or AIDS. Infection with HIV usually leads to AIDS and ultimately death. However, in western countries, most patients survive many years following diagnosis because of the availability of the highly active antiretroviral therapy (HAART). In the absence of HAART, progression from HIV infection to AIDS occurs at a median of between nine to ten years and the median survival time after developing AIDS is only 9.2 months. HAART dramatically increases the time from diagnosis to death, and treatment research continues.
Current optimal HAART options consist of combinations (or "cocktails") consisting of at least three drugs belonging to at least two types, or "classes," of anti-retroviral agents. Typical regimens consist of two nucleoside analogue reverse transcriptase inhibitors (NRTIs) plus either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTI). This treatment is frequently referred to as HAART (highly-active anti-retroviral therapy). Anti-retroviral treatments, along with medications intended to prevent AIDS-related opportunistic infections, have played a part in delaying complications associated with AIDS, reducing the symptoms of HIV infection, and extending patients' life spans. Over the past decade the success of these treatments in prolonging and improving the quality of life for people with AIDS has improved dramatically.
Because HIV disease progression in children is more rapid than in adults, and laboratory parameters are less predictive of risk for disease progression, particularly for young infants, treatment recommendations are more aggressive for children than for adults. In developed countries where HAART is available, doctors assess the viral load, rapidity in CD4 decline, and patient readiness while deciding when to recommend initiating treatment.
There are several concerns about antiretroviral regimens, as side effects of these antiretrovirals have caused problems such as lipodystrophy, dyslipidaemia, insulin resistance, an increase in cardiovascular risks and birth defects. Regimens can be complicated, requiring patients to take several pills at various times during the day, although treatment regimens have been greatly simplified in recent years. If patients miss doses, drug resistance can develop contributing to the rise of viral escape Anti-retroviral drugs are expensive, and the majority of the world's infected individuals do not have access to medications and treatments for HIV and AIDS. Research to improve current treatments includes decreasing side effects of current drugs, further simplifying drug regimens to improve adherence, and determining the best sequence of regimens to manage drug resistance.
A number of studies have shown that measures to prevent opportunistic infections can be beneficial when treating patients with HIV infection or AIDS. Vaccination against hepatitis A and B is advised for patients who are not infected with these viruses and are at risk of getting infected. In addition, AIDS patients should receive vaccination against Streptococcus pneumoniae and should receive yearly vaccination against influenza virus. Patients with substantial immunosuppression are generally advised to receive prophylactic therapy for Pneumocystis jiroveci pneumonia (PCP), and many patients may benefit from prophylactic therapy for toxoplasmosis and Cryptococcus meningitis.
Various forms of alternative medicine have been used to try to treat symptoms or to try to affect the course of the disease itself, although none are a substitute for conventional treatment. In the first decade of the epidemic when no useful conventional treatment was available, a large number of people with AIDS experimented with alternative therapies. The definition of "alternative therapies" in AIDS has changed since that time. Then, the phrase often referred to community-driven treatments, not being tested by government or pharmaceutical company research, that some hoped would directly suppress the virus or stimulate immunity against it. These kinds of approaches have become less common over time as the benefits of AIDS drugs have become more apparent.
Examples of alternative medicine that people hoped would improve their symptoms or their quality of life—include massage, herbal and flower remedies and acupuncture; when used with conventional treatment, many now refer to these as "complementary" approaches. None of these treatments has been proven in controlled trials to have any effect in treating HIV or AIDS directly. However, some may improve feelings of well-being in people who believe in their value. Additionally, people with AIDS, like people with other illnesses such as cancer, sometimes use marijuana to treat pain, combat nausea and stimulate appetite.
Epidemiology
Main article: AIDS pandemic
UNAIDS and the WHO estimate that AIDS has killed more than 25 million people since it was first recognized in 1981, making it one of the most destructive epidemics in recorded history. Despite recent, improved access to antiretroviral treatment and care in many regions of the world, the AIDS epidemic claimed an estimated 3.1 million (between 2.8 and 3.6 million) lives in 2005 of which more than half a million (570,000) were children.
Globally, between 36.7 and 45.3 million people currently live with HIV. In 2005, between 4.3 and 6.6 million people were newly infected and between 2.8 and 3.6 million people with AIDS died, an increase from 2004 and the highest number since 1981.
Sub-Saharan Africa remains by far the worst-affected region, with an estimated 23.8 to 28.9 million people currently living with HIV. More than 60% of all people living with HIV are in sub-Saharan Africa, as are more than three quarters (76%) of all women living with HIV. South & South East Asia are second worst affected with 15%. AIDS accounts for the deaths of 500,000 children.
The latest evaluation report of the World Bank's Operations Evaluation Department assesses the development effectiveness of the World Bank's country-level HIV/AIDS assistance defined as policy dialogue, analytic work, and lending with the explicit objective of reducing the scope or impact of the AIDS epidemic. This is the first comprehensive evaluation of the World Bank's HIV/AIDS support to countries, from the beginning of the epidemic through mid-2004. Because the Bank's assistance is for implementation of government programs by government, it provides important insights on how national AIDS programs can be made more effective.
The development of HAART as effective therapy for HIV infection and AIDS has substantially reduced the death rate from this disease in those areas where it is widely available. This has created the misperception that the disease has gone away. In fact, as the life expectancy of persons with AIDS has increased in countries where HAART is widely used, the number of persons living with AIDS has increased substantially. In the United States, for example, the number of persons with AIDS increased from about 35,000 in 1988 to over 220,000 in 1996.
In the 35 African nations with the highest prevalence, average life expectancy is 48.3 years—6.5 years less than it would be without the disease. For the eleven countries in Africa with prevalence rates above 13%, life expectancy is 47.7 years—11.0 years less than would be expected without HIV/AIDS.
In Africa, the number of MTCT and the prevalence of AIDS is beginning to reverse decades of steady progress in child survival. Countries, such as Uganda are attempting to curb the MTCT epidemic by offering VCT (voluntary counseling and testing), PMTCT (prevention of mother-to-child tranmission) and ANC (ante-natal care) services, which include the distribution of antiretroviral therapy.
Economic impact
HIV and AIDS retard economic growth by destroying human capital. UNAIDS has predicted outcomes for sub-Saharan Africa to the year 2025. These range from a plateau and eventual decline in deaths beginning around 2012 to a catastrophic continual growth in the death rate with potentially 90 million cases of infection.
Without proper nutrition, health care and medicine that is available in developed countries, large numbers of people in these countries are progressing to AIDS. They will not only be unable to work, but will also require significant medical care. The forecast is that this will likely cause a collapse of economies and societies in the region. In some heavily infected areas, the epidemic has left behind many orphans cared for by elderly grandparents.
The increased mortality in this region will result in a smaller skilled population and labour force. This smaller labor force will be predominately young people, with reduced knowledge and work experience leading to reduced productivity. An increase in workers’ time off to look after sick family members or for sick leave will also lower productivity. Increased mortality will also weaken the mechanisms that generate human capital and investment in people, through loss of income and the death of parents. By killing off mainly young adults, AIDS seriously weakens the taxable population, reducing the resources available for public expenditures such as education and health services not related to AIDS resulting in increasing pressure for the state's finances and slower growth of the economy. This then results in slower growth of the tax base, an effect that will be reinforced if there are growing expenditures on treating the sick, training (to replace sick workers) and sick pay and caring for AIDS orphans, especially if the sharp increase in adult mortality shifts the onus from the family to the government in caring for these orphans.
On the level of the household, AIDS results in both the loss of income and increased spending on healthcare by the household. The income effects of this lead to spending reduction as well as a substitution effect away from education and towards healthcare and funeral spending. A study in Côte d'Ivoire showed that households with an HIV/AIDS patient spent twice as much on medical expenses as other households.
UNAIDS, WHO and the United Nations Development Programme have documented a correlation between the decreasing life expectancies and the lowering of gross national product in many African countries with prevalence rates of 10% or more. Indeed, since 1992 predictions that AIDS would slow economic growth in these countries have been published. The degree of impact depended on assumptions about the extent to which illness would be funded by savings and who would be infected. Conclusions reached from models of the growth trajectories of 30 sub-Saharan economies over the period 1990–2025 were that the economic growth rates of these countries would be between 0.56 and 1.47% lower. The impact on gross domestic product (GDP) per capita was less conclusive. However, in 2000, the rate of growth of Africa's per capita GDP was in fact reduced by 0.7% per year from 1990–1997 with a further 0.3% per year lower in countries also affected by malaria. The forecast now is that the growth of GDP for these countries will undergo a further reduction of between 0.5 and 2.6% per annum. However, these estimates may be an underestimate, as they do not look at the effects on output per capita.
Many governments in sub-Saharan Africa denied that there was a problem for years, and are only now starting to work towards solutions. Underfunding is a problem in all areas of HIV prevention when compared to even conservative estimates of the problems.
Stigma
AIDS stigma exists around the world in a variety of ways, including ostracism, rejection, discrimination and avoidance of HIV infected people; compulsory HIV testing without prior consent or protection of confidentiality; violence against HIV infected individuals or people who are perceived to be infected with HIV; and the quarantine of HIV infected individuals.
AIDS stigma has been further divided into the following three categories:
- Instrumental AIDS stigma—a reflection of the fear and apprehension that are likely to be associated with any deadly and transmissible illness.
- Symbolic AIDS stigma—the use of HIV/AIDS to express attitudes toward the social groups or “lifestyles” perceived to be associated with the disease.
- Courtesy AIDS stigma—stigmatization of people connected to the issue of HIV/AIDS or HIV- positive people.
Often, AIDS stigma is expressed in conjunction with one or more other stigmas, particularly those associated with homosexuality, bisexuality, and injection drug use.
In many developed countries, there is still a perceived association between AIDS and homosexuality or bisexuality, and this association is correlated with higher levels of sexual prejudice such as antigay attitudes. There is also a perceived association between all male-male sexual behaviour and AIDS, even sex between two uninfected men. Those most likely to hold misconceptions about HIV transmission and to harbour HIV/AIDS stigma are people with high levels of religiosity, conservative political ideology and less educated people.
- For more details on this topic, see Stigma and HIV-AIDS, A review of the literature
Origin of HIV
Main article: AIDS originThe AIDS epidemic was discovered June 18, 1981, when the U.S. Center for Disease Control and Prevention reported a cluster of Pneumocystis carinii pneumonia (now classified as Pneumocystis jiroveci pneumonia) in five gay men in Los Angeles. Originally dubbed GRID, or Gay-Related Immune Deficiency, health authorities soon realized that nearly half of the people identified with the syndrome were not gay. In 1982, the CDC introduced the term AIDS to describe the newly recognized syndrome.
Three of the earliest known instances of HIV infection are as follows:
- A plasma sample taken in 1959 from an adult male living in what is now the Democratic Republic of Congo.
- HIV found in tissue samples from an American teenager who died in St. Louis in 1969.
- HIV found in tissue samples from a Norwegian sailor who died around 1976.
Two species of HIV infect humans: HIV-1 and HIV-2. HIV-1 is more virulent and more easily transmitted. HIV-1 is the source of the majority of HIV infections throughout the world, while HIV-2 is less easily transmitted and is largely confined to West Africa. Both HIV-1 and HIV-2 are of primate origin. The origin of HIV-1 is the Central Common Chimpanzee (Pan troglodytes troglodytes). It is established that HIV-2 originated from the Sooty Mangabey (Cercocebus atys), an Old World monkey of Guinea Bissau, Gabon, and Cameroon.
Freelance journalist Tom Curtis discussed one currently controversial possibility for the origin of HIV/AIDS in a 1992 Rolling Stone magazine article. He put forward what is now known as the OPV AIDS hypothesis, which suggests that AIDS was inadvertently caused in the late 1950s in the Belgian Congo by Hilary Koprowski's research into a polio vaccine. Although subsequently retracted due to libel issues surrounding its claims, the Rolling Stone article motivated another freelance journalist, Edward Hooper, to probe more deeply into this subject. Hooper's research resulted in his publishing a 1999 book, The River, in which he alleged that an experimental oral polio vaccine prepared using chimpanzee kidney tissue was the route through which SIV crossed into humans to become HIV, thus starting the human AIDS pandemic.
Alternative theories
Main article: AIDS reappraisalA minority of scientists and activists question the connection between HIV and AIDS, or the existence of HIV, or the validity of current testing methods. These claims are met with resistance by, and often evoke frustration and hostility from, most of the scientific community, who accuse the dissidents of ignoring evidence in favour of HIV's role in AIDS, and irresponsibly posing a dangerous threat to public health by their continued activities.
Some assert that the current mainstream approach to AIDS, based on HIV causation, has resulted in inaccurate diagnoses, psychological terror, toxic treatments, and a squandering of public funds. The debate and controversy regarding this issue from the early 1980s to the present has provoked heated emotions and passions from both sides.
A number of misconceptions have arisen surrounding HIV/AIDS. Three of the most common are that AIDS can spread through casual contact, that sexual intercourse with a virgin will cure AIDS, and that HIV can only infect gay men and drug users.
Notes and references
- Marx, J. L. (1982). "New disease baffles medical community". Science. 217 (4560): 618–621. PMID 7089584.
- Gao, F., Bailes, E., Robertson, D. L., Chen, Y., Rodenburg, C. M., Michael, S. F., Cummins, L. B., Arthur, L. O., Peeters, M., Shaw, G. M., Sharp, P. M. and Hahn, B. H. (1999). "Origin of HIV-1 in the Chimpanzee Pan troglodytes troglodytes". Nature. 397 (6718): 436–441. PMID 9989410 doi:10.1038/17130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ UNAIDS (2005). "AIDS epidemic update, 2005" (PDF). Retrieved 2006-01-17. Cite error: The named reference "UNAIDS" was defined multiple times with different content (see the help page).
- Palella, F. J. Jr, Delaney, K. M., Moorman, A. C., Loveless, M. O., Fuhrer, J., Satten, G. A., Aschman and D. J., Holmberg, S. D. (1998). "Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators". N. Engl. J. Med. 338 (13): 853–860. PMID 9516219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morgan, D., Mahe, C., Mayanja, B., Okongo, J. M., Lubega, R. and Whitworth, J. A. (2002). "HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries?". AIDS. 16 (4): 597–632. PMID 11873003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Morgan2" was defined multiple times with different content (see the help page). - ^ Clerici, M., Balotta, C., Meroni, L., Ferrario, E., Riva, C., Trabattoni, D., Ridolfo, A., Villa, M., Shearer, G.M., Moroni, M. and Galli, M. (1996). "Type 1 cytokine production and low prevalence of viral isolation correlate with long-term non progression in HIV infection". AIDS Res. Hum. Retroviruses. 12 (11): 1053–1061. PMID 8827221.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Clerici" was defined multiple times with different content (see the help page). - ^ Morgan, D., Mahe, C., Mayanja, B. and Whitworth, J. A. (2002). "Progression to symptomatic disease in people infected with HIV-1 in rural Uganda: prospective cohort study". BMJ. 324 (7331): 193–196. PMID 11809639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Morgan" was defined multiple times with different content (see the help page). - Gendelman, H. E., Phelps, W., Feigenbaum, L., Ostrove, J. M., Adachi, A., Howley, P. M., Khoury, G., Ginsberg, H. S. and Martin, M. A. (1986). "Transactivation of the human immunodeficiency virus long terminal repeat sequences by DNA viruses". Proc. Natl. Acad. Sci. U. S. A. 83 (24): 9759–9763. PMID 2432602.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bentwich, Z., Kalinkovich., A. and Weisman, Z. (1995). "Immune activation is a dominant factor in the pathogenesis of African AIDS". Immunol. Today. 16 (4): 187–191. PMID 7734046.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tang, J. and Kaslow, R. A. (2003). "The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy". AIDS. 17 (Suppl 4): S51 – S60. PMID 15080180.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Tang" was defined multiple times with different content (see the help page). - Quiñones-Mateu, M. E., Mas, A., Lain de Lera, T., Soriano, V., Alcami, J., Lederman, M. M. and Domingo, E. (1998). "LTR and tat variability of HIV-1 isolates from patients with divergent rates of disease progression". Virus Research. 57 (1): 11–20. PMID 9833881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Campbell, G. R., Pasquier, E., Watkins, J., Bourgarel-Rey, V., Peyrot, V., Esquieu, D., Barbier, P., de Mareuil, J., Braguer, D., Kaleebu, P., Yirrell, D. L. and Loret E. P. (2004). "The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis". J. Biol. Chem. 279 (46): 48197–48204. PMID 15331610.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Campbell" was defined multiple times with different content (see the help page). - Kaleebu P, French N, Mahe C, Yirrell D, Watera C, Lyagoba F, Nakiyingi J, Rutebemberwa A, Morgan D, Weber J, Gilks C, Whitworth J. (2002). "Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda". J. Infect. Dis. 185 (9): 1244–1250. PMID 12001041.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - World Health Organisation (1990). "Interim proposal for a WHO staging system for HIV infection and disease". WHO Wkly Epidem. Rec. 65 (29): 221–228. PMID 1974812.
- ^ CDC (1992). "1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults". CDC. Retrieved 2006-02-09. Cite error: The named reference "MMWR" was defined multiple times with different content (see the help page).
- Holmes, C. B., Losina, E., Walensky, R. P., Yazdanpanah, Y., Freedberg, K. A. (2003). "Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa". Clin. Infect. Dis. 36 (5): 656–662. PMID 12594648.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Guss, D. A. (1994). "The acquired immune deficiency syndrome: an overview for the emergency physician, Part 1". J. Emerg. Med. 12 (3): 375–384. PMID 8040596.
- Guss, D. A. (1994). "The acquired immune deficiency syndrome: an overview for the emergency physician, Part 2". J. Emerg. Med. 12 (4): 491–497. PMID 7963396.
- ^ Schneider, M. F., Gange, S. J., Williams, C. M., Anastos, K., Greenblatt, R. M., Kingsley, L., Detels, R., and Munoz, A. (2005). "Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984-2004". AIDS. 19 (17): 2009–2018. PMID 16260908.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Schneider" was defined multiple times with different content (see the help page). - ^ Lawn, S. D. (2004). "AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection". J. Infect. Dis. 48 (1): 1–12. PMID 14667787. Cite error: The named reference "Lawn" was defined multiple times with different content (see the help page).
- Campbell, G. R., Watkins, J. D., Esquieu, D., Pasquier, E., Loret, E. P. and Spector, S. A. (2005). "The C terminus of HIV-1 Tat modulates the extent of CD178-mediated apoptosis of T cells". J. Biol. Chem. 280 (46): 38376–39382. PMID 16155003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Senkaali, D., Muwonge, R., Morgan, D., Yirrell, D., Whitworth, J. and Kaleebu, P. (2005). "The relationship between HIV type 1 disease progression and V3 serotype in a rural Ugandan cohort". AIDS Res. Hum. Retroviruses. 20 (9): 932–937. PMID 15585080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Feldman, C. (2005). "Pneumonia associated with HIV infection". Curr. Opin. Infect. Dis. 18 (2): 165–170. PMID 15735422.
- Decker, C. F. and Lazarus, A. (2000). "Tuberculosis and HIV infection. How to safely treat both disorders concurrently". Postgrad Med. 108 (2): 57–60, 65–68. PMID 10951746.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Zaidi, S. A. and Cervia, J. S. (2002). "Diagnosis and management of infectious esophagitis associated with human immunodeficiency virus infection". J. Int. Assoc. Physicians AIDS Care (Chic Ill). 1 (2): 53–62. PMID 12942677.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Guerrant, R. L., Hughes, J. M., Lima, N. L., Crane, J. (1990). "Diarrhea in developed and developing countries: magnitude, special settings, and etiologies". Rev. Infect. Dis. 12 (Suppl 1): S41 – S50. PMID 2406855.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Luft, B. J. and Chua, A. (2000). "Central Nervous System Toxoplasmosis in HIV Pathogenesis, Diagnosis, and Therapy". Curr. Infect. Dis. Rep. 2 (4): 358–362. PMID 11095878.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sadler, M. and Nelson, M. R. (1997). "Progressive multifocal leukoencephalopathy in HIV". Int. J. STD AIDS. 8 (6): 351–357. PMID 9179644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gray, F., Adle-Biassette, H., Chrétien, F., Lorin de la Grandmaison, G., Force, G., Keohane, C. (2001). "Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments". Clin. Neuropathol. 20 (4): 146–155. PMID 11495003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Grant, I., Sacktor, H., and McArthur, J. (2005). "HIV neurocognitive disorders". In H. E. Gendelman, I. Grant, I. Everall, S. A. Lipton, and S. Swindells. (ed.) (ed.). The Neurology of AIDS (2nd ed.). London, U.K.: Oxford University Press. pp. 357–373. ISBN 0198526105.
{{cite book}}:|editor=has generic name (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - Satishchandra, P., Nalini, A., Gourie-Devi, M., Khanna, N., Santosh, V., Ravi, V., Desai, A., Chandramuki, A., Jayakumar, P. N., and Shankar, S. K. (2000). "Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96)". Indian J. Med. Res. 11: 14–23. PMID 10793489.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wadia, R. S., Pujari, S. N., Kothari, S., Udhar, M., Kulkarni, S., Bhagat, S., and Nanivadekar, A. (2001). "Neurological manifestations of HIV disease". J. Assoc. Physicians India. 49: 343–348. PMID 11291974.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Boshoff, C. and Weiss, R. (2002). "AIDS-related malignancies". Nat. Rev. Cancer. 2 (5): 373–382. PMID 12044013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Yarchoan, R., Tosatom G. and Littlem R. F. (2005). "Therapy insight: AIDS-related malignancies - the influence of antiviral therapy on pathogenesis and management". Nat. Clin. Pract. Oncol. 2 (8): 406–415. PMID 16130937.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bonnet, F., Lewden, C., May, T., Heripret, L., Jougla, E., Bevilacqua, S., Costagliola, D., Salmon, D., Chene, G. and Morlat, P. (2004). "Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy". Cancer. 101 (2): 317–324. PMID 15241829.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Skoulidis, F., Morgan, M. S., and MacLeod, K. M. (2004). "Penicillium marneffei: a pathogen on our doorstep?". J. R. Soc. Med. 97 (2): 394–396. PMID 15286196.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Donegan, E., Stuart, M., Niland, J. C., Sacks, H. S., Azen, S. P., Dietrich, S. L., Faucett, C., Fletcher, M. A., Kleinman, S. H., Operskalski, E. A.; et al. (1990). "Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations". Ann. Intern. Med. 113 (10): 733–739. PMID 2240875.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Coovadia, H. (2004). "Antiretroviral agents—how best to protect infants from HIV and save their mothers from AIDS". N. Engl. J. Med. 351 (3): 289–292. PMID 15247337. Cite error: The named reference "Coovadia" was defined multiple times with different content (see the help page).
- Kaplan, E. H. and Heimer, R. (1995). "HIV incidence among New Haven needle exchange participants: updated estimates from syringe tracking and testing data". J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10 (2): 175–176. PMID 7552482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ European Study Group on Heterosexual Transmission of HIV (1992). "Comparison of female to male and male to female transmission of HIV in 563 stable couples". BMJ. 304 (6830): 809–813. PMID 1392708.
- ^ Varghese, B., Maher, J. E., Peterman, T. A., Branson, B. M. and Steketee, R. W. (2002). "Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use". Sex. Transm. Dis. 29 (1): 38–43. PMID 11773877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bell, D. M. (1997). "Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview". Am. J. Med. 102 (5B): 9–15. PMID 9845490.
- Leynaert, B., Downs, A. M. and de Vincenzi, I. (1998). "Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV". Am. J. Epidemiol. 148 (1): 88–96. PMID 9663408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rothenberg, R. B., Scarlett, M., del Rio, C., Reznik, D. and O'Daniels, C. (1998). "Oral transmission of HIV". AIDS. 12 (16): 2095–2105. PMID 9833850.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Laga, M., Nzila, N., Goeman, J. (1991). "The interrelationship of sexually transmitted diseases and HIV infection: implications for the control of both epidemics in Africa". AIDS. 5 (Suppl 1): S55 – S63. PMID 1669925.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tovanabutra, S., Robison, V., Wongtrakul, J., Sennum, S., Suriyanon, V., Kingkeow, D., Kawichai, S., Tanan, P., Duerr, A. and Nelson, K. E. (2002). "Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand". J. Acquir. Immune. Defic. Syndr. 29 (3): 275–283. PMID 11873077.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sagar, M., Lavreys, L., Baeten, J. M., Richardson, B. A., Mandaliya, K., Ndinya-Achola, J. O., Kreiss, J. K., and Overbaugh, J. (2004). "Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population". AIDS. 18 (4): 615–619. PMID 15090766.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lavreys, L., Baeten, J. M., Martin, H. L. Jr., Overbaugh, J., Mandaliya, K., Ndinya-Achola, J., and Kreiss, J. K. (2004). "Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study". AIDS. 18 (4): 695–697. PMID 15090778.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cayley, W. E. Jr. (2004). "Effectiveness of condoms in reducing heterosexual transmission of HIV". Am. Fam. Physician. 70 (7): 1268–1269. PMID 15508535.
- WHO (2003). "Condom Facts and Figures". Retrieved 2006-01-17.
- Human Rights Watch (2002). "Ignorance only: HIV/AIDS, Human rights and federally funded abstinence-only programs in the United States. Texas: A case study". Human Rights Watch. Retrieved 2006-03-28.
- The Economist (2005). "Too much morality, too little sense". Retrieved 2006-03-28.
- WHO (2005). "UNAIDS statement on South African trial findings regarding male circumcision and HIV". Retrieved 2006-03-28.
- Various (2005). "Repeated Use of Unsterilized Blades in Ritual Circumcision Might Contribute to HIV Spread in S. Africa, Doctors Say". Kaisernetwork.org. Retrieved 2006-03-28.
- Fan, H. (2005). Fan, H., Conner, R. F. and Villarreal, L. P. eds (ed.). AIDS: science and society (4th ed.). Boston, MA: Jones and Bartlett Publishers. ISBN 076370086X.
{{cite book}}:|editor=has generic name (help); Cite has empty unknown parameter:|chapterurl=(help)CS1 maint: multiple names: editors list (link) - WHO (2003). "WHO, UNAIDS Reaffirm HIV as a Sexually Transmitted Disease". Retrieved 2006-01-17.
- Physicians for Human Rights (2003). "HIV Transmission in the Medical Setting: A White Paper by Physicians for Human Rights". Partners in Health. Retrieved 2006-03-01.
- United Nations General Assembly (2001). "Declaration of Commitment on HIV/AIDS Global Crisis — Global Action". Retrieved 2006-03-01.
- WHO (2001). "Blood safety....for too few". Retrieved 2006-01-17.
- Sperling, R. S., Shapirom D. E., Coombsm R. W., Todd, J. A., Herman, S. A., McSherry, G. D., O'Sullivan, M. J., Van Dyke, R. B., Jimenez, E., Rouzioux, C., Flynn, P. M. and Sullivan, J. L. (1996). "Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant". N. Engl. J. Med. 335 (22): 1621–1629. PMID 8965861.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dias, S. F., Matos, M. G. and Goncalves, A. C. (2005). "Preventing HIV transmission in adolescents: an analysis of the Portuguese data from the Health Behaviour School-aged Children study and focus groups". Eur. J. Public Health. 15 (3): 300–304. PMID 15941747.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Department of Health and Human Services (January, 2005). "A Pocket Guide to Adult HIV/AIDS Treatment January 2005 edition". Retrieved 2006-01-17.
{{cite web}}: Check date values in:|year=(help)CS1 maint: year (link) - Wood, E., Hogg, R. S., Yip, B., Harrigan, P. R., O'Shaughnessy, M. V. and Montaner, J. S. (2003). "Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy?". AIDS. 17 (5): 711–720. PMID 12646794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chene, G., Sterne, J. A., May, M., Costagliola, D., Ledergerber, B., Phillips, A. N., Dabis, F., Lundgren, J., D'Arminio Monforte, A., de Wolf, F., Hogg, R., Reiss, P., Justice, A., Leport, C., Staszewski, S., Gill, J., Fatkenheuer, G., Egger, M. E. and the Antiretroviral Therapy Cohort Collaboration. (2003). "Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies". Lancet. 362 (9385): 679–686. PMID 12957089.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Department of Health and Human Services Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children (November 3, 2005). "Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection" (PDF). Retrieved 2006-01-17.
{{cite web}}: Check date values in:|year=(help)CS1 maint: year (link) - Department of Health and Human Services Panel on Clinical Practices for Treatment of HIV Infection (October 6, 2005). "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents" (PDF). Retrieved 2006-01-17.
{{cite web}}: Check date values in:|year=(help)CS1 maint: year (link) - Montessori, V., Press, N., Harris, M., Akagi, L., Montaner, J. S. (2004). "Adverse effects of antiretroviral therapy for HIV infection". CMAJ. 170 (2): 229–238. PMID 14734438.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Saitoh, A., Hull, A. D., Franklin, P. and Spector, S. A. (2005). "Myelomeningocele in an infant with intrauterine exposure to efavirenz". J. Perinatol. 25 (8): 555–556. PMID 16047034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dybul, M., Fauci, A. S., Bartlett, J. G., Kaplan, J. E., Pau, A. K.; Panel on Clinical Practices for Treatment of HIV. (2002). "Guidelines for using antiretroviral agents among HIV-infected adults and adolescents". Ann. Intern. Med. 137 (5 Pt 2): 381–433. PMID 12617573.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Becker, S., Dezii, C. M., Burtcel, B., Kawabata, H. and Hodder, S. (2002). "Young HIV-infected adults are at greater risk for medication nonadherence". MedGenMed. 4 (3): 21. PMID 12466764.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Saltmarsh, S. (2005). "Voodoo or valid? Alternative therapies benefit those living with HIV". Positively Aware. 3 (16): 46. PMID 16479668.
- Mills, E., Wu, P. and Ernst, E. (2005). "Complementary therapies for the treatment of HIV: in search of the evidence". Int. J. STD AIDS. 16 (6): 395–403. PMID 15969772.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - World Bank (2005). "Evaluating the World Bank's Assistance for Fighting the HIV/AIDS Epidemic". Retrieved 2006-01-17.
- CDC (1996). "U.S. HIV and AIDS cases reported through December 1996" (PDF). HIV/AIDS Surveillance Report. 8 (2): 1–40.
- ^ Greener, R. (2002). "AIDS and macroeconomic impact". In S, Forsyth (ed.) (ed.). State of The Art: AIDS and Economics. IAEN. pp. 49–55.
{{cite book}}:|editor=has generic name (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - Bollinger, L. and Stover, J. (1999). "The Economic Impact of AIDS" (PDF). iaen. Retrieved 2006-03-28.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Over, M. (1992). "The macroeconomic impact of AIDS in Sub-Saharan Africa, Population and Human Resources Department". The World Bank.
- Bonnel, R. (2000). "HIV/AIDS and Economic Growth: A Global Perspective". S. A. J. Economics. 68 (5): 820–855.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - Bell, C., Gersbach, H. and Devarajan, S. (2003). "The long-run economic costs of AIDS: theory and an application to South Africa". eldis. Retrieved 2006-03-28.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Herek, G. M. and Capitanio, J. P. (1999). "AIDS Stigma and sexual prejudice" (PDF). Am. Behav, Scientist. Retrieved 2006-03-27.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Snyder M, Omoto AM, Crain AL. (1999). "Punished for their good deeds: stigmatization for AIDS volunteers". Am. J. Public Health. 42 (7): 1175–1192.
{{cite journal}}: Cite has empty unknown parameter:|1=(help)CS1 maint: multiple names: authors list (link) - Herek GM, Capitanio JP, Widaman KF. (2002). "HIV-related stigma and knowledge in the United States: prevalence and trends, 1991-1999" (PDF). Am. J. Public Health. 92 (3): 371–377.
{{cite journal}}: Text "PMID 11867313" ignored (help)CS1 maint: multiple names: authors list (link) - Herek, GM, Widaman, KF, Capitanio, JP (2005). "When sex equals AIDS: Symbolic stigma and heterosexual adults' inaccurate beliefs about sexual transmission of AIDS" (PDF). Social Problems. 52 (1): 15–37.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - United States Health Resources and Services Administration. "Stigma and HIV-AIDS, A review of the literature". HRSA. Retrieved 2006-03-24.
- CDC (1981). "Pneumocystis Pneumonia --- Los Angeles". CDC. Retrieved 2006-01-17.
- Zhu, T., Korber, B. T., Nahmias, A. J., Hooper, E., Sharp, P. M. and Ho, D. D. (1998). "An African HIV-1 Sequence from 1959 and Implications for the Origin of the Epidemic". Nature. 391 (6667): 594–597. PMID 9468138 doi:10.1038/35400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Reeves, J. D. and Doms, R. W (2002). "Human Immunodeficiency Virus Type 2". J. Gen. Virol. 83 (Pt 6): 1253–1265. PMID 12029140.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Curtis, T. (1992). "The origin of AIDS". Rolling Stone (626): 54–59, 61, 106, 108.
- Hooper, E. (1999). The River : A Journey to the Source of HIV and AIDS (1st ed.). Boston, MA: Little Brown & Co. pp. 1–1070. ISBN 0316372617.
- Duesberg, P. H. (1988). "HIV is not the cause of AIDS". Science. 241 (4865): 514, 517. PMID 3399880.
- Papadopulos-Eleopulos, E., Turner, V. F., Papadimitriou, J., Page, B., Causer, D., Alfonso, H., Mhlongo, S., Miller, T., Maniotis, A. and Fiala, C. (2004). "A critique of the Montagnier evidence for the HIV/AIDS hypothesis". Med Hypotheses. 63 (4): 597–601. PMID 15325002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cohen, J. (1994). "The Duesberg phenomenon" (PDF). Science. 266 (5191): 1642–1644. PMID 7992043.
External links
HIV/AIDS resources:
- AEGiS.org AIDS Education Global Information System
- AVERT AIDS Education & Research Trust from the U.K
- Eldis HIV and AIDS - latest research and other resources on HIV and AIDS in developing countries
- AIDS.ORG: Comprehensive HIV/AIDS Information
- HIV/AIDS glossary from the U. S. Department of Health and Human Services
- AIDSmeds.com: Comprehensive lessons on HIV/AIDS and their treatments
- The Body: The Complete HIV/AIDS Resource
- AIDS facts and news from the National Institute of Allergy and Infectious Diseases
- HIV and AIDS from New Scientist
- AIDS treatment news
- AIDS Newspaper Articles Archive
HIV/AIDS associations and programmes:
- The Joint United Nations Programme on HIV/AIDS (UNAIDS)
- International AIDS Society - the world's leading independent association of HIV/AIDS professionals
- Evaluating the World Bank's Assistance for Fighting the HIV/AIDS Epidemic
- Health Action: HIV Transmission in the Medical Setting
Support groups and activism:
- Elizabeth Glaser Pediatric AIDS Foundation
- World AIDS Day World AIDS Day 1 December
- Student global AIDS campaign
- Elton John AIDS Foundation
- Mercury Phoenix Trust
Documentaries:
- Documentation on the oral polio vaccine (OPV) theory of AIDS origin AIDSOrigin.com
- Documentary about AIDS in Africa: Living with AIDS
- Origin of Aids Video Watch Free online : Origin of Aids Video
- Recorded interview with Prof. Robert Gallo about the progress in HIV vaccine research
Dissident viewpoints:
- AIDS Wiki's comprehensive list of dissident websites
- The Body: AIDS Denialism and list of resources criticizing the "AIDS reappraisal" movement
Template:Link FA Template:Link FA Template:Link FA
Categories: