| Revision as of 09:23, 17 April 2012 editMartinvl (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers18,715 edits →References: Adding Notes section← Previous edit | Revision as of 18:49, 18 April 2012 edit undoMartinvl (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers18,715 edits →Impact on reproducibility: Rewrite of sectionNext edit → | ||
| Line 203: | Line 203: | ||
| :'''Proposed definition:''' The candela, cd, is the unit of luminous intensity in a given direction; its magnitude is set by fixing the numerical value of the luminous efficacy of monochromatic radiation of frequency {{val|540|e=12|u=Hz}} to be equal to exactly 683 when it is expressed in the unit s<sup>3</sup>·m<sup>−2</sup>·kg<sup>−1</sup>·cd·sr, or cd·sr·W<sup>−1</sup>, which is equal to lm·W<sup>−1</sup>. | :'''Proposed definition:''' The candela, cd, is the unit of luminous intensity in a given direction; its magnitude is set by fixing the numerical value of the luminous efficacy of monochromatic radiation of frequency {{val|540|e=12|u=Hz}} to be equal to exactly 683 when it is expressed in the unit s<sup>3</sup>·m<sup>−2</sup>·kg<sup>−1</sup>·cd·sr, or cd·sr·W<sup>−1</sup>, which is equal to lm·W<sup>−1</sup>. | ||
| In 1889 the GCPM took delivery of 40 prototype metres and 40 prototype kilograms from the British firm ]. One of each prototype was retained by the CGPM as the international prototype, a set of working copies were retained by the BIPM and the rest were distributed to member nations for use as their national prototypes. At regular intervals the national prototypes were compared with and recalibrated against the international prototype.<ref>{{cite journal | |||
| |last1= Jabbour | |||
| |first1= Z.J. | |||
| |last2= Yaniv | |||
| |first2= S.L. | |||
| |year= 2001 | |||
| |title= The Kilogram and Measurements of Mass and Force | |||
| |journal= J. Res. Natl. Inst. Stand. Technol. | |||
| |volume= 106 | |||
| |issue= 1 | |||
| |pages= 25–46 | |||
| |publisher= ] (NIST | |||
| |url= http://nvl.nist.gov/pub/nistpubs/jres/106/1/j61jab.pdf | |||
| |accessdate= 2011-03-28}}</ref> | |||
| In 1921 the Convention of the metre was revised and the mandate of the CGPM was extended to provide standards for all units of measure, not just mass and length. In the ensuing years the CGPM took on responsibility for providing standards of ], ], ], ] and ].<ref>{{SIbrochure8th|pages=95, 97}}</ref> | |||
| ==Impact on reproducibility== | ==Impact on reproducibility== | ||
| Overall the changes in the definitions will give an improvement in the uncertainty of the reproduction of the base units using the standard ''mise en pratique'' (practical technique). | |||
| In the New SI, none of the base units will defined directly. Apart from the candela,<ref group="Note" name="CandelaNote"/> all the base will be defined in terms of universal physical constants thus six physical constnats will be needed to define the six base units. When the New SI was first designed, there were more than six suitable physical constants from which the designers could choose, for example, once length and time had been established, the ] ''G'' was a suitable candidate from a dimensional point of view to define mass,<ref group="Note">The dimensions of ''G'' are L<sup>3</sup>M<sup>-1</sup>T<sup>-2</sup>, so once standards have been established for length and for time, mass can in theory be deduced from ''G''.</ref> but was unsuitable as in practice it can only be measured an uncertainty of 10<sup>-4</sup><ref name=CODATA>{{cite web | |||
| The following table catalogues the improvements:<ref name="draft"/><ref>{{cite web | |||
| |url = http://www.codata.org/taskgroups/TGfundconst/ | |||
| |title = CODATA Fundemental Physical Constants | |||
| |publisher = International Council for Science : Committee on Data for Science and Technology(CODATA) | |||
| |year = 2011 | |||
| |accessdate = 17 April 2012}}</ref> whereas the international prototype kilogram can be measured with an uncertainty of 5 × 10<sup>-8</sup>. The choice was made on the basis of minimal uncertainty associated with measuring the constant and the degree of independence of the constant in respect of other constants that were being used. Although the BIPM has developed a standard ''mise en pratique'' (practical technique)<ref>{{cite web | |||
| |url = http://www.bipm.org/en/si/new_si/mise-en-pratique.html | |||
| |title = What is a ''mise en pratique''? | |||
| |publisher = ] | |||
| |date = 2011 | |||
| |accessdate = 2011-03-11}}</ref> for each type of measurement, the ''mise and practique'' used to make the measurement is not part of the measurement's definition - it is merely an assurance that the measurement can be done with a specified maximum uncertainty. | |||
| ===Fundamental physical constants=== | |||
| The following table catalogues the uncertainties of the fundamental constants of nature used to define the base units of SI using the 2006 CODATA least squares adjustment.<ref name=CODATA/> The relative uncertainties are expected to improve as time progresses. | |||
| {| class="wikitable" style="text-align: center;" | |||
| |- bgcolor="#efefef" | |||
| !colspan="3"|Relative uncertainty of fundamental constants | |||
| |- | |||
| !Constant | |||
| !Symbol | |||
| !Uncertainty<br>(× 10<sup>−9</sup>) | |||
| |- | |||
| |]<br> of the caesium 133 atom | |||
| |Δν(<sup>133</sup>Cs)<sub>hfs</sub> | |||
| |1 × 10<sup>−5</sup> | |||
| |- | |||
| |] | |||
| |''c'' | |||
| |25 | |||
| |- | |||
| |] | |||
| |''h'' | |||
| |50 | |||
| |- | |||
| |] | |||
| |''e'' | |||
| |25 | |||
| |- | |||
| |] | |||
| |''k'' | |||
| |1700 | |||
| |- | |||
| |] | |||
| |''N''<sub>A</sub> | |||
| |1.4 | |||
| |} | |||
| ===Base units=== | |||
| The uncertainty of all of the SI units depends on the uncertainty with which the fundamental units on which the base units depend can be measured - the uncertainty of the base units themselves depends only on experimental limitations. The uncertainties of the base units using the existing definitions techniques compared to the uncertainty with what they would be using the new definitions are catalogued below:<ref name="MillsNote"/> | |||
| {| class="wikitable" style="text-align: center;" | |||
| |- bgcolor="#efefef" | |||
| !colspan="5"|Relative uncertainty of base units | |||
| |- | |||
| !Unit | |||
| !Symbol | |||
| !Under current<br>definitions (× 10<sup>−9</sup>) | |||
| !Under proposed<br>definitions (× 10<sup>−9</sup>) | |||
| |- | |||
| |second<ref name = Penzes>{{cite web | |||
| |url = http://www.nist.gov/pml/div681/upload/museum-timeline.pdf | |||
| |title = Time Line for the Definition of the Meter | |||
| |author = William B. Penzes | |||
| |accessdate = 2011-01-01}}</ref> | |||
| |s | |||
| |1 × 10<sup>−5</sup> | |||
| |<ref group="Note" name=NoChange>Unaffected by the changes in definition.</ref> | |||
| |- | |||
| |metre<ref name = Penzes/> | |||
| |m | |||
| |25 | |||
| |<ref group="Note" name=NoChange/> | |||
| |- | |||
| |mass | |||
| |kg | |||
| |50 | |||
| |50 | |||
| |- | |||
| |ampere | |||
| |A | |||
| |25 | |||
| |0.69 | |||
| |- | |||
| |kelvin | |||
| |K | |||
| |1700 | |||
| |1700 | |||
| |- | |||
| |Molar mass <sup>12</sup>C | |||
| |''M''(<sup>12</sup>C) | |||
| |1.4 | |||
| |1.4 | |||
| |- | |||
| |candela<ref>{{cite web | |||
| |url = http://www.npl.co.uk/reference/measurement-units/si-base-units/the-candela | |||
| |title = The candela (cd) | |||
| |publisher = ] (NPL) | |||
| |location = ], United Kingdom | |||
| |year = 2010 | |||
| |accessdate = 16 April 2012}}</ref><ref group="Note" name="CandelaNote">Measurement of the candela also requires a knowledge of the response of the human eye (]) to different wavelengths of light.</ref> | |||
| |''cd'' | |||
| |2 × 10<sup>6</sup> | |||
| |<ref group="Note" name=NoChange/> | |||
| |} | |||
| ===Other physical constants=== | |||
| There are three categories of physical constants: | |||
| * The fundamental constants whose value is by definition fixed. The uncertainties associated with these constants have already been catalogued. | |||
| * Physical constants that are a function of the fundamental constants, for example the von Klitzing constant ''R''<sub>K</sub> = ''h/e<sup>2</sup>''. In this case, both ''e'' and ''h'' are fundamental constants, so the von Klitzing constant has an exact definition. | |||
| * Physical constants that were alternative candidates as fundamental constants. These have to be measured separately, but the fundamental constants are often used in calculating these constants. | |||
| Although there are potentially many thousand of constants in the latter two groups, those identified by Mills<ref name="MillsNote">{{cite web | |||
| |url = http://www1.bipm.org/cc/CIPM/Allowed/98/CIPM2009_49_TIMING_THE_NEW_SI.pdf | |url = http://www1.bipm.org/cc/CIPM/Allowed/98/CIPM2009_49_TIMING_THE_NEW_SI.pdf | ||
| |title = A Note to the CIPM from Ian Mills, President of the CCU: Thoughts about the timing of the change from the Current SI to the New SI | |title = A Note to the CIPM from Ian Mills, President of the CCU: Thoughts about the timing of the change from the Current SI to the New SI | ||
| Line 213: | Line 340: | ||
| |date = October 2010 | |date = October 2010 | ||
| |publisher = CCU | |publisher = CCU | ||
| |accessdate = 2011-01-01}}</ref> | |accessdate = 2011-01-01}}</ref> are listed below. Constants that are closely related have been grouped together.<ref name="draft"/> | ||
| {| class="wikitable" style="text-align: center;" | {| class="wikitable" style="text-align: center;" | ||
| |- bgcolor="#efefef" | |- bgcolor="#efefef" | ||
| !colspan=" |
!colspan="4"|Relative uncertainty of various physical measurements | ||
| |- | |- | ||
| !Unit | |||
| !Constant used as reference | !Constant used as reference | ||
| !Symbol | !Symbol | ||
| Line 225: | Line 350: | ||
| !Proposed definitions | !Proposed definitions | ||
| |- | |- | ||
| |electron mass<br>] or ]<br>carbon 12 atomic mass | |||
| |rowspan="2"|kg | |||
| |''m''<sub>e</sub><br>''m''<sub>u</sub><br>''m''(<sup>12</sup>C) | |||
| |Mass of International prototype kilogram | |||
| |5.0 × 10<sup>−8</sup><br>5.0 × 10<sup>−8</sup><br>5.0 × 10<sup>−8</sup><br> | |||
| |''m''(K) | |||
| |1.4 × 10<sup>−9</sup><br>1.4 × 10<sup>−9</sup><br>1.4 × 10<sup>−9</sup> | |||
| |exact | |||
| |5.0 × 10<sup>−8</sup> | |||
| |- | |- | ||
| |]<br>]<br>] | |||
| |Planck constant | |||
| |''μ''<sub>0</sub><br>''ε''<sub>0</sub><br>Z<sub>0</sub> | |||
| |''h'' | |||
| |exact<br>exact<br>exact | |||
| |5.0 × 10<sup>−8</sup> | |||
| |6.9 × 10<sup>−10</sup><br>6.8 × 10<sup>−10</sup><br>6.8 × 10<sup>−10</sup> | |||
| |exact | |||
| |- | |- | ||
| |] | |||
| |rowspan="2"|A | |||
| |α | |||
| |magnetic constant | |||
| | |
|6.8 × 10<sup>−10</sup> | ||
| |6.8 × 10<sup>−10</sup> | |||
| |exact | |||
| |6.9 × 10<sup>−10</sup> | |||
| |- | |- | ||
| |] | |||
| |Elementary charge | |||
| |'' |
|''R''<sub>K</sub> | ||
| | |
|6.8 × 10<sup>−10</sup> | ||
| |exact | |exact | ||
| |- | |- | ||
| |] | |||
| |rowspan="2"|K | |||
| |Temperature of triple point of water | |||
| |''T''<sub>TPW</sub> | |''T''<sub>TPW</sub> | ||
| |exact | |exact | ||
| |1.7 × 10<sup>−6</sup> | |1.7 × 10<sup>−6</sup> | ||
| |- | |- | ||
| | |
|] | ||
| |'' |
|''R'' | ||
| |1.7 × 10<sup>−6</sup> | |1.7 × 10<sup>−6</sup> | ||
| |exact | |exact | ||
| |- | |- | ||
| |] | |||
| |rowspan="2"|mol | |||
| |α | |||
| |Molar mass <sup>12</sup>C | |||
| | |
| 700 × 10<sup>−6</sup> | ||
| |exact | |||
| |1.4 × 10<sup>−9</sup> | |||
| |- | |||
| |Avogadro constant | |||
| |''N''<sub>A</sub> | |||
| |1.4 × 10<sup>−9</sup> | |||
| |exact | |exact | ||
| |- | |- | ||
| |]<br>] | |||
| |''F''<br>''K<sub>J</sub> | |||
| |25<br>25 | |||
| |exact<br>exact | |||
| |} | |} | ||
| The relative uncertainty in |
The relative uncertainty in the mass of International Prototype Kilogram (''m(K)'') under the proposed system will be | ||
| 50 × 10<sup>−9</sup>. | |||
| |url = http://www.nist.gov/pml/div681/upload/museum-timeline.pdf | |||
| |title = Time Line for the Definition of the Meter | |||
| |author = William B. Penzes | |||
| |accessdate = 2011-01-01}}</ref> | |||
| ==Criticisms of the proposed new SI definitions== | ==Criticisms of the proposed new SI definitions== | ||
Revision as of 18:49, 18 April 2012

A committee of the International Committee for Weights and Measures (CIPM) has proposed revised formal definitions of the SI base units, which are being examined by the CIPM and which may be considered by the 25th CGPM, in 2014.
In summary, the changes are proposed as follows:
- "There will still be the same seven base units (second, metre, kilogram, ampere, kelvin, mole, and candela). Of these, the kilogram, ampere, kelvin and mole will be redefined by choosing exact numerical values for the Planck constant, the elementary electric charge, the Boltzmann constant, and the Avogadro constant, respectively. The second, metre and candela are already defined by physical constants and it is only necessary to edit their present definitions. The new definitions will improve the SI without changing the size of any units, thus ensuring continuity with present measurements."
Further details are found in the draft chapter of the Ninth SI Units Brochure.
The last major overhaul of the metric system was in 1960 when the International System of Units (SI) was formally published as a coherent set of units of measure. SI is structured around seven base units that have apparently "arbitrary" definitions and another twenty units that are derived from these base units. Although the units themselves form a coherent system, the definitions do not. The proposals before the CIPM seek to remedy this by using the fundamental quantities of nature as the basis for deriving the base units. This will mean, amongst other things, archiving the prototype kilogram. The second and the metre are already defined in such a manner.
There have been numerous criticisms of the revised definitions since their initial proposal, and it has been argued that the proposal for the new SI requires frank and open discussion before decisions are made.
Background to the proposal of new SI definitions
In 1875, twenty of the most industrially developed nations of the world met for the Convention of the Metre. The result was the signing of the Treaty of the Metre under which three bodies were set up to regulate units of measure that were to be used internationally. They were:
- CGPM (General Conference on Weights and Measures / Conférence Générale des Poids et Mesures) – The Conference meets every four to six years and consists of representatives of the nations who had signed the convention. It discusses and examines the arrangements required to ensure the propagation and improvement of the International System of Units and it endorses the results of new fundamental metrological determinations.
- CIPM (International Committee for Weights and Measures/Comité international des poids et mesures) The Committee consists of eighteen eminent scientists, each from a different country, nominated by the CGPM. The CIPM meets annually and is tasked to advise the CGPM. The CIPM has set up a number of sub-committees, each charged with a particular area of interest. One of these, the CCU (Consultative Committee for Units), amongst other things, advises the CIPM on matters concerning units of measurement.
- BIPM (International Bureau for Weights and Measures/Bureau international des poids et mesures) – The Bureau provides laboratory facilities and in the secretariat for the CIPM and the CGPM.
In 1889 the GCPM took delivery of 40 prototype metres and 40 prototype kilograms from the British firm Johnson Matthey. One of each of these was nominated by lot as the international prototypes, other copies were retained by the CGPM as working copies and the rest were distributed to member nations for use as their national prototypes. At regular intervals the national prototypes were compared with and recalibrated against the international prototype.
In 1921 the Convention of the metre was revised and the mandate of the CGPM was extended to provide standards for all units of measure, not just mass and length. In the ensuing years the CGPM took on responsibility for providing standards of time, electric current, temperature, molar mass and luminosity.
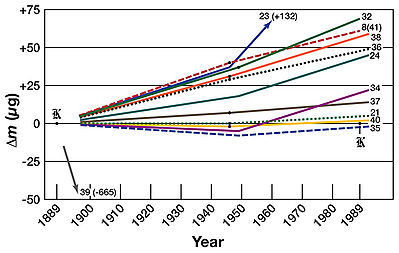
Since 1960, when the definition of the metre was linked to a particular wavelength of light rather than the international prototype metre, the only unit of measure that has been dependent on a particular artifact has been the kilogram. Over the years, small drifts which could be as high as 20×10 kilograms per annum in the mass of the international prototype kilogram have been detected. At the 21st meeting of the GCPM (1999), national laboratories were urged to investigate ways of breaking the link between the kilogram and a specific artifact.
A report published in 2007 by the Consultative Committee for Thermometry to the CIPM noted that their current definition of temperature has proved to be unsatisfactory for temperatures below 20 K and for temperatures above 1300 K. The committee was of the view that the Boltzmann constant provided a better basis for temperature measurement than did the triple point of water, as it overcame these difficulties.
At its 23rd meeting (2007), the GCPM mandated the CIPM to investigate the use of natural constants as the basis for all units of measure rather than the artifacts that were then in use. The following year this was endorsed by the International Union of Pure and Applied Physics (IUPAP). At a meeting of the CCU held in Reading, United Kingdom in September 2010, a resolution and draft changes to the SI brochure that were to be presented to the next meeting of the CIPM in October 2010 were agreed to in principle. The CIPM meeting of October 2010 found that "the conditions set by the General Conference at its 23rd meeting have not yet been fully met. For this reason the CIPM does not propose a revision of the SI at the present time"; however the CIPM presented a resolution for consideration at the 24th CGPM (17 - 21 October 2011) to agree the new definitions in principle, but not to implement them until the details have been finalised. This resolution was accepted by the conference, and in addition the CGPM moved the date of the 25th meeting forward from 2015 to 2014.
Mohr, in a paper that that discussed the CGPM proposal but which predated it, suggested that since the proposed system makes use of atomic scale phenomena rather than macroscopic phenomena, it should be called the "Quantum SI System".
The proposals
- In this section, an "X" at the end of a number means that the final digit is yet to be agreed upon.
The CCU has proposed that in addition to the speed of light, four constants of nature be defined to have exact values:
- Planck's constant h is exactly 6.62606X×10joule second (J·s).
- An elementary charge e is exactly 1.60217X× 10coulomb (C).
- Boltzmann constant k is exactly 1.38065X×10joule per kelvin (J·K).
- Avogadro constant NA is exactly 6.02214X×10reciprocal mole (mol).
These constants were described in the 2006 version of the SI manual; the latter three were defined as "constants to be obtained by experiment".
It is proposed that the numerical values associated with the following constants of nature be retained unchanged:
- The speed of light c is exactly 299792458metres per second (m·s).
- The ground state hyperfine splitting frequency of the caesium-133 atom Δν(Cs)hfs is exactly 9192631770 hertz (Hz).
- The luminous efficacy Kcd of monochromatic radiation of frequency 540×10Hz is exactly 683 lumen per watt (lm·W).
The seven definitions above are rewritten below after converting the derived units (joule, coulomb, hertz, lumen and watt) into the seven base units (second, metre, kilogram, ampere, kelvin, mole and candela). In the list that follows, the symbol sr stands for the dimensionless unit steradian.
- Δν(Cs)hfs = 9192631770s
- c = 299792458s·m
- h = 6.62606X×10s·m·kg
- e = 1.60217X×10s·A
- k = 1.38065X×10s·m·kg·K
- NA = 6.02214X×10mol
- Kcd = 683 s·m·kg·cd·sr
In addition the CCU has proposed that:
These changes will have the effect of redefining the SI base units, though the definitions of the derived SI units will remain the same.
Proposed changes to the base units
It is proposed that the text of the definitions of all the base units be either refined or rewritten. The current (2008) and proposed (2011) definitions are given below. In many cases the final digit of any constant is yet to be agreed, so it has been represented by an "X"

Second
The proposed definition is effectively the same as the current definition, the only difference being that the conditions under which the measurements are made more rigorous.
- Current definition: The second is the duration of 9192631770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium-133 atom.
- Proposed definition: The second, s, is the unit of time; its magnitude is set by fixing the numerical value of the ground state hyperfine splitting frequency of the caesium-133 atom, at rest and at a temperature of 0 K, to be equal to exactly 9192631770 when it is expressed in the unit s, which is equal to Hz.
Metre
The proposed definition is effectively the same as the current definition, the only difference being that the tightening up of the definition of the second will propagate to the metre
- Current definition: The metre is the length of the path travelled by light in vacuum during a time interval of 1/299792458 of a second.
- Proposed definition: The metre, m, is the unit of length; its magnitude is set by fixing the numerical value of the speed of light in vacuum to be equal to exactly 299792458 when it is expressed in the unit m·s.
Kilogram
The definition of the kilogram is undergoing a fundamental change - the current definition defines the kilogram as being the mass of the international prototype kilogram, the new definition relates it to the equivalent energy of a photon via Planck's constant.
- Current definition: The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram.
- Proposed definition: The kilogram, kg, is the unit of mass; its magnitude is set by fixing the numerical value of the Planck constant to be equal to exactly 6.62606X×10 when it is expressed in the unit s·m·kg, which is equal to J·s.
One consequence of this change is that the new definition makes the definition of the kilogram dependent on the definitions of the second and the metre.
Ampere
The definition of the ampere is undergoing a major overhaul—the current definition, which is difficult to realise with high precision in practice, is being replaced by a definition which is more intuitive and which is easier to realise in practice.
- Current definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 m apart in vacuum, would produce between these conductors a force equal to 2×10 newton per metre of length.
- Proposed definition: The ampere, A, is the unit of electric current; its magnitude is set by fixing the numerical value of the elementary charge to be equal to exactly 1.60217X×10 when it is expressed in the unit A·s, which is equal to C.
One consequence of this change is that the new definition of the Ampere will not be dependent on the definitions of the kilogram and the metre. In addition, by fixing the elementary charge to an exact value, the vacuum permeability, vacuum permittivity and impedance of free space, which are currently exact along with the speed of light, will all consequently carry experimental error.
Kelvin
The definition of the kelvin will undergo a fundamental change if the proposals are accepted. Rather than using points where water changes state to fix the temperature scale the proposal recommends that the energy equivalent as given by Boltzmann's equation be used.
- Current definition: The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
- Proposed definition: The kelvin, K, is the unit of thermodynamic temperature; its magnitude is set by fixing the numerical value of the Boltzmann constant to be equal to exactly 1.38065X×10 when it is expressed in the unit s·m·kg K, which is equal to J·K.
One consequence of this change is that the new definition makes the definition of the kelvin depend on the definitions of the second, the metre, and the kilogram.
Mole
The current definition of the mole links it to the kilogram. The proposed definition will break that link by making a mole a specific number of entities of the substance in question.
- Current definition: The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
- Proposed definition: The mole, mol, is the unit of amount of substance of a specified elementary entity, which may be an atom, molecule, ion, electron, any other particle or a specified group of such particles; its magnitude is set by fixing the numerical value of the Avogadro constant to be equal to exactly 6.02214X×10 when it is expressed in the unit mol.
One consequence of this change is that the current defined relationship between the mass of the C atom, the dalton, the kilogram, and Avogadro's number will no longer be valid. One of the following must change:
- the mass of C, which would no longer be exactly 12 dalton
- the mass of the dalton itself. This would change the numerical masses of all atoms except C
- the number of C atoms in 12 grams or 0.012 kilogram, which is currently NA by definition.
Candela
The proposed definition is effectively the same as the current definition, but rephrased.
- Current definition: The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540×10 Hz and that has a radiant intensity in that direction of 1/683 watt per steradian.
- Proposed definition: The candela, cd, is the unit of luminous intensity in a given direction; its magnitude is set by fixing the numerical value of the luminous efficacy of monochromatic radiation of frequency 540×10 Hz to be equal to exactly 683 when it is expressed in the unit s·m·kg·cd·sr, or cd·sr·W, which is equal to lm·W.
In 1889 the GCPM took delivery of 40 prototype metres and 40 prototype kilograms from the British firm Johnson Matthey. One of each prototype was retained by the CGPM as the international prototype, a set of working copies were retained by the BIPM and the rest were distributed to member nations for use as their national prototypes. At regular intervals the national prototypes were compared with and recalibrated against the international prototype.
In 1921 the Convention of the metre was revised and the mandate of the CGPM was extended to provide standards for all units of measure, not just mass and length. In the ensuing years the CGPM took on responsibility for providing standards of time, electric current, temperature, molar mass and luminosity.
Impact on reproducibility
In the New SI, none of the base units will defined directly. Apart from the candela, all the base will be defined in terms of universal physical constants thus six physical constnats will be needed to define the six base units. When the New SI was first designed, there were more than six suitable physical constants from which the designers could choose, for example, once length and time had been established, the universal gravitational constant G was a suitable candidate from a dimensional point of view to define mass, but was unsuitable as in practice it can only be measured an uncertainty of 10 whereas the international prototype kilogram can be measured with an uncertainty of 5 × 10. The choice was made on the basis of minimal uncertainty associated with measuring the constant and the degree of independence of the constant in respect of other constants that were being used. Although the BIPM has developed a standard mise en pratique (practical technique) for each type of measurement, the mise and practique used to make the measurement is not part of the measurement's definition - it is merely an assurance that the measurement can be done with a specified maximum uncertainty.
Fundamental physical constants
The following table catalogues the uncertainties of the fundamental constants of nature used to define the base units of SI using the 2006 CODATA least squares adjustment. The relative uncertainties are expected to improve as time progresses.
| Relative uncertainty of fundamental constants | ||
|---|---|---|
| Constant | Symbol | Uncertainty (× 10) |
| ground state hyperfine splitting frequency of the caesium 133 atom |
Δν(Cs)hfs | 1 × 10 |
| speed of light | c | 25 |
| Planck constant | h | 50 |
| Elementary charge | e | 25 |
| Boltzmann constant | k | 1700 |
| Avogadro constant | NA | 1.4 |
Base units
The uncertainty of all of the SI units depends on the uncertainty with which the fundamental units on which the base units depend can be measured - the uncertainty of the base units themselves depends only on experimental limitations. The uncertainties of the base units using the existing definitions techniques compared to the uncertainty with what they would be using the new definitions are catalogued below:
| Relative uncertainty of base units | ||||
|---|---|---|---|---|
| Unit | Symbol | Under current definitions (× 10) |
Under proposed definitions (× 10) | |
| second | s | 1 × 10 | ||
| metre | m | 25 | ||
| mass | kg | 50 | 50 | |
| ampere | A | 25 | 0.69 | |
| kelvin | K | 1700 | 1700 | |
| Molar mass C | M(C) | 1.4 | 1.4 | |
| candela | cd | 2 × 10 | ||
Other physical constants
There are three categories of physical constants:
- The fundamental constants whose value is by definition fixed. The uncertainties associated with these constants have already been catalogued.
- Physical constants that are a function of the fundamental constants, for example the von Klitzing constant RK = h/e. In this case, both e and h are fundamental constants, so the von Klitzing constant has an exact definition.
- Physical constants that were alternative candidates as fundamental constants. These have to be measured separately, but the fundamental constants are often used in calculating these constants.
Although there are potentially many thousand of constants in the latter two groups, those identified by Mills are listed below. Constants that are closely related have been grouped together.
| Relative uncertainty of various physical measurements | |||
|---|---|---|---|
| Constant used as reference | Symbol | Current definitions | Proposed definitions |
| electron mass unified atomic mass unit or dalton carbon 12 atomic mass |
me mu m(C) |
5.0 × 10 5.0 × 10 5.0 × 10 |
1.4 × 10 1.4 × 10 1.4 × 10 |
| magnetic constant vacuum permittivity impedance of free space |
μ0 ε0 Z0 |
exact exact exact |
6.9 × 10 6.8 × 10 6.8 × 10 |
| Hall effect constant | α | 6.8 × 10 | 6.8 × 10 |
| von Klitzing constant | RK | 6.8 × 10 | exact |
| temperature of triple point of water | TTPW | exact | 1.7 × 10 |
| Molar gas constant | R | 1.7 × 10 | exact |
| Stefan–Boltzmann constant | α | 700 × 10 | exact |
| Faraday constant Josephson constant |
F KJ |
25 25 |
exact exact |
The relative uncertainty in the mass of International Prototype Kilogram (m(K)) under the proposed system will be 50 × 10.
Criticisms of the proposed new SI definitions
Price has argued that the new proposal will:
- cause confusion because the new explicit-constant definitions do not relate a unit to an example of its quantity
- risk damage to the enterprise of science because the circular definition of units will render it impossible to detect any future change in fundamental constants
- cause economic damage due to increased transaction costs and barriers to international trade
Leonard has argued that "the fundamental concept of the mole requires the number of entities comprising one mole, i.e. Avogadro's number, to be exactly equal to the gram-to-dalton mass ratio" and that the proposal breaks this compatibility condition by defining the kilogram, dalton and mole independently.
Pavese has argued that a number of issues need to be better understood before the definitions are changed. The issues include the count nature and the value of the Avogadro number; the loss of the concept of base unit; the possibility of checking future changes in the 'fundamental constants', and the shift to the unit of the experimental uncertainty.
See also
External links
- BIPM website on the New SI, including a FAQ page.
- The New SI: Units of measurement based on fundamental constants
Notes
- Prototype No. 8(41) was accidentally stamped with the number 41, but its accessories carry the proper number 8. Since there is no prototype marked 8, this prototype is referred to as 8(41).
- ^ Measurement of the candela also requires a knowledge of the response of the human eye (luminosity function) to different wavelengths of light.
- The dimensions of G are LMT, so once standards have been established for length and for time, mass can in theory be deduced from G.
- ^ Unaffected by the changes in definition.
References
- ^
"On the possible future revision of the International System of Units, the SI" (Document). Sèvres, France: International Bureau for Weights and Measures. 21 Oct, 2011.
{{cite document}}: Check date values in:|date=(help); Unknown parameter|contribution=ignored (help); Unknown parameter|url=ignored (help) It is not expected to be adopted until some prerequisite conditions are met, and in any case not before 2014. See "Possible changes to the international system of units". IUPAC Wire. 34 (1). International Union of Pure and Applied Chemistry. January–February 2012.{{cite journal}}: CS1 maint: date format (link) - Peter Mohr (November 2, 2011). "Redefining the SI base units". NIST Newsletter. NIST. Retrieved 2012-03-01.
-
Michael Kuehne (05 December 2012). "Redefinition of the SI". Keynote address, ITS (Ninth International Temperature Symposium}. Los Angeles: NIST. Retrieved 2012-03-01.
{{cite web}}: Check date values in:|date=(help) - Ian Mills (27 September 2010). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). BIPM. Retrieved 2012-03-01.
- "CIPM: International Committee for Weights and Measures". BIPM. Retrieved 2010-10-03.
- Jabbour, Z.J.; Yaniv, S.L. (2001). "The Kilogram and Measurements of Mass and Force" (PDF). J. Res. Natl. Inst. Stand. Technol. 106 (1). National Institute of Standards and Technology (NIST: 25–46. Retrieved 2011-03-28.
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), pp. 95, 97, ISBN 92-822-2213-6, archived (PDF) from the original on 2021-06-04, retrieved 2021-12-16
- G. Girard (1994). "The Third Periodic Verification of National Prototypes of the Kilogram (1988–1992)". Metrologia. 31 (4): 317–336. Bibcode:1994Metro..31..317G. doi:10.1088/0026-1394/31/4/007.
- Peter Mohr (6 December 2010). "Recent progress in fundamental constants and the International System of Units" (PDF). Third Workshop on Precision Physics and Fundamental Physical Constants. Retrieved 2011-01-02.
- Fischer, J.; et al. (2 May 2007). "Report to the CIPM on the implications of changing the definition of the base unit kelvin" (PDF). Retrieved 2011-01-02.
{{cite web}}: Explicit use of et al. in:|author=(help) - "Resolution proposal submitted to the IUPAP Assembly by Commission C2 (SUNAMCO)" (PDF). International Union of Pure and Applied Physics. 2008. Retrieved 2011-05-08.
- Ian Mills (29 September 2010). "On the possible future revision of the International System of Units, the SI" (PDF). CCU. Retrieved 2011-01-01.
- ^ Ian Mills (29 September 2010). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). CCU. Retrieved 2011-01-01.
- "Towards the "new SI"". International Bureau of Weights and Measures (BIPM). Retrieved 2011-02-20.
- "On the possible future revision of the International System of Units, the SI - Draft Resolution A" (PDF). International Committee for Weights and Measures (CIPM). Retrieved 2011-07-14.
- "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. 23 October 2011. Retrieved 25 October 2011.
- Mohr, Peter J (2008). "The Quantum SI: A Possible New International System of Units". Advances in Quantum Chemistry. Academic Press: 34. ISBN 978-0-12-373925-4. Retrieved 2 April 2012.
{{cite journal}}: Unknown parameter|vol=ignored (|volume=suggested) (help) - Jabbour, Z.J.; Yaniv, S.L. (2001). "The Kilogram and Measurements of Mass and Force" (PDF). J. Res. Natl. Inst. Stand. Technol. 106 (1). National Institute of Standards and Technology (NIST: 25–46. Retrieved 2011-03-28.
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), pp. 95, 97, ISBN 92-822-2213-6, archived (PDF) from the original on 2021-06-04, retrieved 2021-12-16
- ^ "CODATA Fundemental Physical Constants". International Council for Science : Committee on Data for Science and Technology(CODATA). 2011. Retrieved 17 April 2012.
- "What is a mise en pratique?". BIPM. 2011. Retrieved 2011-03-11.
- ^ Ian Mills (October 2010). "A Note to the CIPM from Ian Mills, President of the CCU: Thoughts about the timing of the change from the Current SI to the New SI" (PDF). CCU. Retrieved 2011-01-01.
- ^ William B. Penzes. "Time Line for the Definition of the Meter" (PDF). Retrieved 2011-01-01.
- "The candela (cd)". Teddington, United Kingdom: National Physical Laboratory (NPL). 2010. Retrieved 16 April 2012.
- Price, Gary (2011). "A skeptic's review of the New SI". Accreditation and Quality Assurance: Journal for Quality, Comparability and Reliability in Chemical Measurement. 16 (3): 121–132. doi:10.1007/s00769-010-0738-x.
{{cite journal}}:|access-date=requires|url=(help) - Leonard, B. P. (2010). "Comments on recent proposals for redefining the mole and kilogram". Metrologia. 47 (3): L5 – L8. doi:10.1088/0026-1394/47/3/L01.
- Pavese, Franco (2011). "Some reflections on the proposed redefinition of the unit for the amount of substance and of other SI units". Accreditation and Quality Assurance: Journal for Quality, Comparability and Reliability in Chemical Measurement. 16 (3): 161–165. doi:10.1007/s00769-010-0700-y.
{{cite journal}}:|access-date=requires|url=(help)
| SI units | |
|---|---|
| Base units | |
| Derived units with special names | |
| Other accepted units | |
| See also | |