| Revision as of 20:17, 21 August 2012 edit24.185.202.30 (talk) →Question about oral sex: trolling... if not, medical advice← Previous edit | Revision as of 20:25, 21 August 2012 edit undoStuRat (talk | contribs)Extended confirmed users, Pending changes reviewers88,546 edits Undid revision 508508800 by 24.185.202.30 (talk) I think medical advice, so will mark it as such.Next edit → | ||
| Line 586: | Line 586: | ||
| :The nearest that I could find to an official webpage is , which uses ''"Castleton Botanical Garden"'' in its header, although just to confuse matters it calls it ''"Castelton Gardens"'' in the following text. ] (]) 19:36, 21 August 2012 (UTC) | :The nearest that I could find to an official webpage is , which uses ''"Castleton Botanical Garden"'' in its header, although just to confuse matters it calls it ''"Castelton Gardens"'' in the following text. ] (]) 19:36, 21 August 2012 (UTC) | ||
| == Question about oral sex == | |||
| Does the eating of semen affect your health in any way? Please answer as soon as possible, I've read that it causes cancer and I've been doing it for a while and it's ruined it all for me, I'm afraid of it now. <small><span class="autosigned">— Preceding ] comment added by ] (] • ]) 20:06, 21 August 2012 (UTC)</span></small><!-- Template:Unsigned --> <!--Autosigned by SineBot--> | |||
Revision as of 20:25, 21 August 2012
Welcome to the science sectionof the Misplaced Pages reference desk. skip to bottom Select a section: Shortcut Want a faster answer?
Main page: Help searching Misplaced Pages
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Misplaced Pages:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
August 17
what's the squiggly side group symbol stand for?
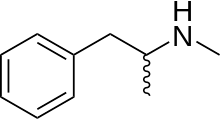
See the structural diagram for Methamphetamine. What does the squiggly side group stand for? μηδείς (talk) 00:47, 17 August 2012 (UTC)
- See skeletal formula to learn what a bond being a wavy line means. DMacks (talk) 00:52, 17 August 2012 (UTC)
- Thanks, that gives a general answer of unknown or stereoisomer. In this case its obviously a stereoisomer of some chiral sidegroup. Can anyone specify it? μηδείς (talk) 01:02, 17 August 2012 (UTC)
- The active form is dextrorotary, which in this case is "out of the screen". Bizarrely, Levomethamphetamine is also drug, but functions only as a decongestant. Someguy1221 (talk) 01:05, 17 August 2012 (UTC)When I wrote this comment, I thought you were asking which isomer it was, not which sidegroup it was. Someguy1221 (talk) 01:17, 17 August 2012 (UTC)
- So, the squiggly side group is a methyl group. Is that what you're asking? 203.27.72.5 (talk) 01:10, 17 August 2012 (UTC)
- That it is a methyl group is clear from this image. 203.27.72.5 (talk) 01:14, 17 August 2012 (UTC)
- Otherwise unannotated squiggly lines are always methyl groups, just as otherwise unannotated straight lines are always methyl groups. Someguy1221 (talk) 01:16, 17 August 2012 (UTC)
- Okay, so it's a methyl group and a hydrogen then, since a methyl alone would require a missing double bond. Makes sense. I don't remember the squiggly side group. When did that convention come into style? I remember a wedge perhaps next to a line, or an R. μηδείς (talk) 01:19, 17 August 2012 (UTC)
- The wedges represent specific isomers. The squiggle represents a racemic mixture. And if it is a methyl group there must be a hydrogen. If there were a double bond it would not be a methyl group. See methyl group. 203.27.72.5 (talk) 01:23, 17 August 2012 (UTC)
- It occurred to me that you might mean a double bond like this; R-CH=C(CH2)R, rather then like this; R-CH2-CH(=CH2)R as I first assumed. If that's the case then the methyl group would still be indicated by a line even though there's no hydrogen there. So the line doesn't represent the hydrogen as well, just the CH3. The hydrogens are ommited by convention. 203.27.72.5 (talk) 01:45, 17 August 2012 (UTC)
- Yes, I did mean what you first assumed, 203. And at this point I fully get what is symbolized for this specific molecule. And I agree that there would have to be a hydrogen for the remaining side group if none other were signified. My point is, I do not remember the squiggly mark from Organic Chem when I tested in or took it. For the Bio part of my undergrad double-major I tested out of OC lecture in the early nineties. (Before that I got A's in Chem I & II and Biochem in high school and a V on the AP test.) And I aced organic chem lab when I finally took it in the mid nineties. I simply do not remember the squiggly convention. Should it be interpreted as any stereoisomeric sidegroup? (Any old racemic mixture?) And when did the squiggly convention begin? I am concerned that I had an Ivy League education with a sub-par Org Chem class. μηδείς (talk) 02:45, 17 August 2012 (UTC)
- Okay, so it's a methyl group and a hydrogen then, since a methyl alone would require a missing double bond. Makes sense. I don't remember the squiggly side group. When did that convention come into style? I remember a wedge perhaps next to a line, or an R. μηδείς (talk) 01:19, 17 August 2012 (UTC)
- Otherwise unannotated squiggly lines are always methyl groups, just as otherwise unannotated straight lines are always methyl groups. Someguy1221 (talk) 01:16, 17 August 2012 (UTC)
- The active form is dextrorotary, which in this case is "out of the screen". Bizarrely, Levomethamphetamine is also drug, but functions only as a decongestant. Someguy1221 (talk) 01:05, 17 August 2012 (UTC)When I wrote this comment, I thought you were asking which isomer it was, not which sidegroup it was. Someguy1221 (talk) 01:17, 17 August 2012 (UTC)
- Thanks, that gives a general answer of unknown or stereoisomer. In this case its obviously a stereoisomer of some chiral sidegroup. Can anyone specify it? μηδείς (talk) 01:02, 17 August 2012 (UTC)
- To quote the page I pointed you to earlier, "wavy lines represent either unknown stereochemistry or a mixture of the two possible stereoisomers at that point". I know I've seen it in literature from at least back to the mid-1980s, but I don't know the history of IUPAC or others officially blessing it. DMacks (talk) 03:14, 17 August 2012 (UTC)
- Here it is being used in 1989, so it at least predates your college days as far as I can see. To be fair though, I don't think it was ever explicity pointed out by my lecturers when I was at uni, and I majored in chem. It was just something I picked up on from seeing examples where that was the only possible meaning. 203.27.72.5 (talk) 03:17, 17 August 2012 (UTC)
- I found these earlier examples but they all seem a bit odd to me. I'm not 100% sure that they're using the squiggly line in the same way that we do these days. 203.27.72.5 (talk) 04:42, 17 August 2012 (UTC)
- I made the change to methamphetamine and someone quickly pointed out that the squiggle actually just means undefined, not racemic in any particular ratio. I should have thought of that, but I'll point it out now. Wnt (talk) 16:27, 17 August 2012 (UTC)
I very highly doubt the textbook edition I used in '90 that had been published in 1987 or 1989 was using a convention that we cannot find much prior to that date--textbooks tend to be conservative. I will accept this question as resolved. μηδείς (talk) 07:02, 18 August 2012 (UTC)
ET contact book
The question above about extraterrestrials reminded me of a book I read about on Misplaced Pages, and that I'm hoping someone could identify. The plot is that an enormous alien spacecraft has entered the solar system on a fast hyperbolic trajectory. Due to celestial mechanics, the only human spacecraft capable of docking with it is piloted by a young girl on her first solo flight (an average Joe in some ways), but her ship wouldn't have enough fuel to return to the solar system. The rest of the book describes her exploration of the mysterious artificial ecosystem inside the alien craft, which apparently had water, but many other features were extremely bizarre and non-Earth-like. At the end, there was no cheesy cop-out, and the girl did starve (or die some other way) on board, but the book was really more about the alien craft than about the explorer.
Before anyone mentions it, this is not Rendezvous with Rama (which is a great book, by the way; I highly recommend it). It seems to share a similar plot, but then again there are millions of books about aliens out there. --68.179.115.177 (talk) 06:43, 17 August 2012 (UTC)
- Yeah Rendezvous with Rama was my first thought. I'd love to know too, sounds like a great read.-- OBSIDIAN†SOUL 07:29, 17 August 2012 (UTC)
- I found something like it. Pushing Ice by Alastair Reynolds. Though in this case, it was an entire crew of a mining ship, but the captain is still female.-- OBSIDIAN†SOUL 09:32, 17 August 2012 (UTC)
- The description reminds me somewhat of a short story by James Tiptree, Jr. It may have been the lead title (and certainly inspired the pbk cover illustration) of one of her short story collections, but I'm afraid I can't remember which and, being at work, can't consult my library. Hopefully this might give someone a helpful lead. {The poster formerly known as 87.81.230.195} 84.21.143.150 (talk) 12:57, 17 August 2012 (UTC)
- False lead, mea culpa. I was misremembering "The Only Neat Thing to Do", whose plot is not close enough to that sought to be a candidate. {The poster formerly known as 87.81.230.195} 90.197.66.109 (talk) 21:15, 17 August 2012 (UTC)
- Man, I hope someone finds this. I would like to read this, sounds interesting!
Which jobs require sharp vision?
I am endowed with exceptionally sharp vision, which enables me among else to read signs from a long distance. My motor skills are normal, and not as developed as those of an air pilot or a sniper. Which civilian jobs require sharp vision, besides relevant academic education? Thanks, 93.172.151.222 (talk) 10:36, 17 August 2012 (UTC)
- Something in forensics, maybe? I don't think there are really many jobs where better than normal vision is particularly useful. --Tango (talk) 12:10, 17 August 2012 (UTC)
- Off the top of my head: On the floor of the stock exchange; Any captain of a passenger vehicle (cruise ship, airliner, bus, etc.); Operator of heavy machinery (crane, bulldozer, etc.); Janitor; Quality control.165.212.189.187 (talk) 15:55, 17 August 2012 (UTC)
- Private eye ? There one particular vision skill is needed, at which I seem rather deficient: facial recognition. (I can only recognize people in context. If I met my mother someplace unexpected, and she didn't talk, I wouldn't recognize her.) StuRat (talk) 15:54, 17 August 2012 (UTC)
- That sounds like as a disability. Qpl87 (talk) 16:07, 17 August 2012 (UTC)
- In general (not commenting on any one case) I suspect that prosopagnosia, or more properly prosopamnesia, is uncommonly common on Misplaced Pages... Wnt (talk) 16:49, 17 August 2012 (UTC)
- Cool, so now I can get a handicapped sticker and park up front. :-) StuRat (talk) 16:51, 17 August 2012 (UTC)
- I'd say referee, but maybe sharp vision there would be a handicap. :) Also the security freaks who watch people at casinos and such? Wnt (talk) 17:10, 17 August 2012 (UTC)
Airline pilot? Sniper? Sniper spotter? 203.27.72.5 (talk) 21:15, 17 August 2012 (UTC)
- Congratulations on your excellent vision, (this is from a resentful myopic) but I can't help noticing that all the occupations suggested need a good many other skills apart from good vision. Your exceptional visual acuity needs to be combined with sound judgement, knowledge, morality and experience, gather those 4 friends around you and you are on your way. Richard Avery (talk) 06:39, 18 August 2012 (UTC)
- How are you at hitting a baseball? Ted Williams supposedly had 20/10 vision, which supposedly was helpful in his ability to hit. ←Baseball Bugs carrots→ 05:07, 19 August 2012 (UTC)
physics/5th Dimension
I don't understand what is defined as 5th Dimension ? I know the space and time is considered as Third and Forth dimension, but not sure about Fifth one? What the picture shows actually ?How can we observe/feel the 5th dimension?
--Kesavan (talk) 13:09, 17 August 2012 (UTC)
- First off lets pretend we are 2D (length and width) beings on the surface of a balloon (here time is the 3rd dimension). A spot on the other side of the balloon is a distance away, as we have to stick to the surface of the balloon. However, as we have 3 spacial dimensions (and a time dimension) to work with, we can take a "short cut" though the centre of the balloon, shortening the distance between the 2 points. So this is one way a 4th spacial dimension would revel itself, particles arriving at places sooner than expected. A second time dimension I'm not sure on how that would appear Dja1979 (talk) 14:38, 17 August 2012 (UTC).
- This diagram, by itself, does not contain any useful information. It is part of a longer
paperletter-to-the-editor-of-a-physics-journal, On the dimensionality of spacetime, available from its author's website: Max Tegmark, professor of Physics at MIT. Have you read his paper? It explains the author's views about space and time. In my opinion, these views are not particularly rigorous, nor are the explanations of the assumptions particularly sound; but, nobody other than the author is responsible for his reasoning. Nimur (talk) 17:18, 17 August 2012 (UTC)
- This diagram, by itself, does not contain any useful information. It is part of a longer
- "we are here " seems to me to be a bit short sighted because it only refers to our physical selves. When I look at the three dimensions of time and one of space I think of past, present and future, then I think what can travel in these dimensions? A: thoughts.... my thoughts can travel back in time and recall real events and that "information" since it is obviously not lost is still "somewhere" in my head. So is that information traveling ahead into the future to meet my consciousness in the present? Or do I have some intrinsic ability to travel at least in one dimension of thought to the past?GeeBIGS (talk) 02:39, 18 August 2012 (UTC)
- when I think of one dimension of both space and time I think of light, two dimensions of space and one of time I think of the plane between positive and negative charge in a magnet, one dimension of time by itself or space without the other is like a singularityGeeBIGS (talk) 02:48, 18 August 2012 (UTC)
- The information in your mind travels into the future along with your body. You do not travel back in time when you consider memories; you are only activating symbolic representations of past events. —Tamfang (talk) 01:26, 19 August 2012 (UTC)
- "we are here " seems to me to be a bit short sighted because it only refers to our physical selves. When I look at the three dimensions of time and one of space I think of past, present and future, then I think what can travel in these dimensions? A: thoughts.... my thoughts can travel back in time and recall real events and that "information" since it is obviously not lost is still "somewhere" in my head. So is that information traveling ahead into the future to meet my consciousness in the present? Or do I have some intrinsic ability to travel at least in one dimension of thought to the past?GeeBIGS (talk) 02:39, 18 August 2012 (UTC)
- Kesavan, the chart is a summary of what (the author thinks) existence would be like if the universe had a different number of dimensions. As it says, "we are here": our universe has exactly three spatial and one temporal dimension, so a fifth doesn't correspond to anything.
- That's not to say, though, that five (or more) dimensions can never be useful. Any system with five continuous independent variables can be represented by a five-dimensional continuum; but these dimensions have no necessary relation to the 3+1 dimensions of our universe's spacetime. —Tamfang (talk) 01:26, 19 August 2012 (UTC)
- In a hypothetical 4+1D universe, with one more spatial dimension than we do, the fifth dimension would be time. In a hypothetical 5+1D universe, with two more spatial dimensions than we do, the fifth dimension would be a spatial dimension. But we live in a 3+1D universe (well, on the macroscopic scale), and there aren't enough dimensions for the term "fifth dimension" to mean anything here.
- But the fifth dimension is useful. See Kaluza-Klein theory and five-dimensional space. And of course, mathematically speaking, five-dimensional space is extremely useful if you are a 5-polytope. ;-) Double sharp (talk) 13:03, 19 August 2012 (UTC)
Approximately how much mass does the Milky Way lose each day?
Due to stars radiating energy into extragalactic space. Assume no mass is being gained from external sources. Goodbye Galaxy (talk) 14:22, 17 August 2012 (UTC)
- and I just realized how appropriate my username is for this question
- Since you ask only about radiated light, this is an easy calculation. Orders of magnitude (power) claims that the Milky Way radiates 5×10 W (though it doesn't cite a source). Divided by c, that's 5×10 kg/day. -- BenRG (talk) 18:02, 17 August 2012 (UTC)
- Which is remarkably close to the mass of Earth. That seems very small to me... just goes to show how big the speed of light is, I guess. --Tango (talk) 19:35, 17 August 2012 (UTC)
- Ok, but how much does it recieve each day from intergalactic space? SkyMachine 03:39, 18 August 2012 (UTC)
- Since the Galaxy is mostly empty space, I imagine that most mass "received" simply passes right through and keeps going. Someguy1221 (talk) 03:47, 18 August 2012 (UTC)
- The net balance is ~zero, but can anyone estimate the daily incoming energy transaction? SkyMachine 03:53, 18 August 2012 (UTC)
- I disagree that the net balance is about zero. As the universe gets older and moves towards heat death, the amount of energy that exists outside galaxies in the form on photons (and other particles) moving through intergalactic space increases. That means galaxies are, on average and on balance, losing energy. As Someguy says, most of the energy from outside the galaxy that enters it never actually interacts (other than gravitationally) with it and just passes straight through. The actual energy absorbed by the galaxy from outside will be minimal. --Tango (talk) 20:26, 18 August 2012 (UTC)
- The net balance is ~zero, but can anyone estimate the daily incoming energy transaction? SkyMachine 03:53, 18 August 2012 (UTC)
- By net balance I meant the difference in the ammount of energy originating from outside the galaxy that enters and leaves the milky way at any point in time. An analogy if you like: if a high rung meth dealer gets $100k each day as a result of drug dealing but spends ~$100k on hookers, cocaine, casino gambling, protection, and bribes, his net monetary gain is zero but alot of money is going through his hands on any given day. I want to know the energy influx from outside the galaxy that enters the milky way at any given time. SkyMachine 03:42, 19 August 2012 (UTC)
- Yes, I know what "net" means and I disagree with you for the reasons stated. --Tango (talk) 12:01, 19 August 2012 (UTC)
- By net balance I meant the difference in the ammount of energy originating from outside the galaxy that enters and leaves the milky way at any point in time. An analogy if you like: if a high rung meth dealer gets $100k each day as a result of drug dealing but spends ~$100k on hookers, cocaine, casino gambling, protection, and bribes, his net monetary gain is zero but alot of money is going through his hands on any given day. I want to know the energy influx from outside the galaxy that enters the milky way at any given time. SkyMachine 03:42, 19 August 2012 (UTC)
Is an apple alive or dead when you eat it?
Three questions in one:
1. At the instant before you take the first bite of an apple is it considered alive or dead?
2. At any moment during consumption of an apple is it considered alive or dead?
3. After you've finished eating an apple, is the remaining apple core considered alive or dead?
Thank you for your time. — Preceding unsigned comment added by Wallywalrus (talk • contribs) 15:43, 17 August 2012 (UTC)
- It's difficult to define alive or dead in plants. I will use the definition "it is alive if it can grow a new apple tree". In that case, the apple, or more specifically the seeds, are still alive after all three steps (assuming the seeds were viable to begin with). In fact, even if you ate the seeds, they might still be viable after you poop them out. StuRat (talk) 15:49, 17 August 2012 (UTC)
- (ec) Technically one could say the apple is alive, in the sense that it can produce an apple tree. However, the only 'living' part are the seeds, the rest of the apple is actually food meant to be eaten, because when animals eat the apple, they unintentionally spread the seeds around, to the advantage of the apple tree that produced the apple. Certainly by eating an apple you do not 'kill' it, afterwards it has not lost its ability to grow (and eventually reproduce). - Lindert (talk) 15:51, 17 August 2012 (UTC)
What are alive in any organism are its cells. A fruit (excluding its seeds) is not an organism, but a part of one. The skin of an apple consists of live photosynthesizing cells which allow it to grow and produce various oils and pigments as the fruit matures. They remain living for some time after the fruit matures. There is not very much I can find at wikipedia, but see pome and exocarp. μηδείς (talk) 17:52, 17 August 2012 (UTC)
- In multi-cellular animals, the organism can be dead even when most of its cells are still alive. This would be the case, for example, right after somebody was beheaded. Some of the cells with low metabolic rates (like those which grow hair), can stay alive for quite some time. However, with plants, there really isn't this distinction between the life of the cells and the organism. The plant is basically "just the sum of its cells". StuRat (talk) 18:02, 17 August 2012 (UTC)
- Snopes and other debunkers deny old stories of fingernails and hair continuing to grow after death. When you die, your hair and fingernail production stops, but the flesh may dehydrate, exposing more of the hair or nails. Edison (talk) 03:03, 18 August 2012 (UTC)
- Yes, but those cells don't die the very second your heart stops. StuRat (talk) 03:06, 18 August 2012 (UTC)
- If they did, organ transplants wouldn't be possible. Organs such as hearts, livers, and kidneys can "survive" for several hours outside the body, if treated properly.Sjö (talk) 06:51, 18 August 2012 (UTC)
- And a fruit is not an organism, so there is no sense in asking if it, as opposed to some of its parts, is alive. The apple is not alive as such, but many of its cells are, for quite a long time. So long as the skin is both open to air and not brown or frozen you should view its cells as alive. μηδείς (talk) 06:56, 18 August 2012 (UTC)
- But, as previously explained, the concept of an organism being alive or dead, as opposed to the cells, only applies to multi-cellular animals. So, asking if an apple tree is alive is about the same as asking if an apple is alive. With plants, perhaps asking "Is it still viable ?" would be the better question. StuRat (talk) 07:02, 18 August 2012 (UTC)
- You could use that terminology, in which case apple skin itself is not viable, even though it can be cultured in a lab and produce a thallus. Otherwise the seeds, which are usually considered distinct, and not consumed, would be the only part normally considered viable. Given that the seeds are not eaten, but the skin is, and it is alive, I would stick with yes as the answer. μηδείς (talk) 07:13, 18 August 2012 (UTC)
- I read somewhere that most fruit you buy at a supermarket are hybrid varieties and as such sterile, anyway.Уга-уга12 (talk) 21:05, 21 August 2012 (UTC)
- This adds yet another wrinkle. Certainly we can't consider the entire sterile plant to be dead, just because it can't reproduce. So, I suggest the rule there should be "consider it to be alive if it could reproduce, were it not sterile". StuRat (talk) 21:25, 21 August 2012 (UTC)
Different magnets
Is there an article in here about non-metallic magnetic materials? Is it possible to make a magnet which would repel the Earth? Is is possible to have a "laser" magnet? — Preceding unsigned comment added by Qpl87 (talk • contribs) 16:01, 17 August 2012 (UTC)
- Plastic magnet is about non-metallic magnets. To repel the Earth an enormous and very heavy magnet would be required, but theoretically it is possible. I'm not sure what you mean by a 'laser magnet'. - Lindert (talk) 16:10, 17 August 2012 (UTC)
- By "laser" magnet, I meant a magnet that instead of having a round field, would have a narrow field, concentrating its force on one point. Qpl87 (talk) 16:27, 17 August 2012 (UTC)
- No, unless a magnetic monopole is discovered, magnetic fields are necessarily round, because according to Gauss's law for magnetism, total magnetic flux into any volume is zero. - Lindert (talk) 16:46, 17 August 2012 (UTC)
- By "laser" magnet, I meant a magnet that instead of having a round field, would have a narrow field, concentrating its force on one point. Qpl87 (talk) 16:27, 17 August 2012 (UTC)
- And why is a magnet that repels the Earth necessarily enormous and very heavy? According to your link polymers can be magnetic, so theoretically, should it be possible to construct a light-weight plastic magnet that would float around? Qpl87 (talk) 16:49, 17 August 2012 (UTC)
- Yes, but to mainly repel the Earth instead of being repelled by it, it would need a lot of mass. If you are not concerned about getting the Earth moving, but moving away from the Earth, I guess you might use a lighter magnet. Anyway, I highly doubt any permanent magnet can be created in practice that is more repelled by the Earth's magnetic field than it is attracted by the Earth's gravity. - Lindert (talk) 17:09, 17 August 2012 (UTC)
- Well any magnet can repel the Earth, it just can't repel it very much. :) Wnt (talk) 17:12, 17 August 2012 (UTC)
- Technically, they repel each other by an equal amount, but, of course, the more massive object moves far less. StuRat (talk) 18:11, 17 August 2012 (UTC)
- Magnetic fields are not "necessarily round", Gauss's law only states that the total flux through a closed surface is zero. See: Halbach array, Maxwell coil.—eric 18:19, 17 August 2012 (UTC)
- Not round in the sense of prefectly circular, but magnetic field lines always form a loop (like in the Halbach array). - Lindert (talk) 18:26, 17 August 2012 (UTC)
- So, back to the OP's question, yes, it is possible to have a narrow magnetic field, but I wouldn't describe such a field as a "laser magnet". 203.27.72.5 (talk) 20:49, 17 August 2012 (UTC)
- This is all a bit dubious. The north poles of two magnets repel and likewise the south poles do the same. But if a magnet was sufficiently powerful that it could levitate by it's north pole repeling the earth's south pole, the magnet's south pole would be so attracted that it would change it's orientation towards the earth. You would need some mechanism to constantly keep the magnet in a particular orientation. If you want to achieve leviation, you'd be better off exploiting the Meissner effect with a superconductor, though I don't understand enough about this to know if it would ever work. 203.27.72.5 (talk) 20:44, 17 August 2012 (UTC)
- Theoretically, if two magnets are perfectly aligned, neither should flip. However, placing a shaft between the two magnets will ensure that they don't (or just through the magnet you want to levitate above the Earth, stuck into the ground). StuRat (talk) 20:49, 17 August 2012 (UTC)
- That's incorrect. See Earnshaw's theorem. 203.27.72.5 (talk) 03:19, 18 August 2012 (UTC)
- There's no reason the alignment shaft shouldn't work. See Pseudo-levitation#Mechanical_constraint_.28pseudo-levitation.29. As for getting it to work without a mechanical constraint, that can be done with diamagnetism. StuRat (talk) 05:53, 18 August 2012 (UTC)
- Yes, that part is alright, but "Theoretically, if two magnets are perfectly aligned, neither should flip." is wrong. And as for diamagnetism, that' exactly what I was suggesting with superconductors. 203.27.72.5 (talk) 07:46, 18 August 2012 (UTC)
- That part is also fine, so long as one is a diamagnetic, so I'm not sure what you're disagreeing with. StuRat (talk) 10:18, 18 August 2012 (UTC)
- No, that statement is wrong since it doesn't specify that one be a diamagnet, and if it did, it would be nonsensical, because the repulsion of a diamagnet and a magnetic field is independent of alignment. 203.27.72.5 (talk) 20:27, 18 August 2012 (UTC)
- "Wrong" and "not specific" aren't the same thing. And the diamagnet would need to be aligned. If off-center from the object generating the magnetic field, it would fall off the edge. StuRat (talk) 21:25, 18 August 2012 (UTC)
- Right, so "all cars are red and blue" isn't wrong, it's just not specific that I'm only talking about Domino's Pizza delivery cars. And in this case the object generating the magnetic field is earth, so how does something fall off? 203.27.72.5 (talk) 21:53, 18 August 2012 (UTC)
- I didn't say "all"', and I wasn't talking about the Earth at that point. StuRat (talk) 23:33, 18 August 2012 (UTC)
- I suppose you could do it with a gyroscope, or a very small motor with fast-reacting computer control, but by what margin would we need to improve on existing magnets to get one that levitates in this way? Wnt (talk) 23:40, 17 August 2012 (UTC)
- You could make a blimp that's just slightly heavier than air, including an electromagnet, then use the electromagnet to lift off the ground. I don't see this being a very practical means of transportation, though. StuRat (talk) 02:06, 18 August 2012 (UTC)
- Apparently, even that wouldn't work. See . I tried to calculate the field strength of a magnet that would be required to cancel out the force of gravity, but I ran into...er...complications. 203.27.72.5 (talk) 03:59, 18 August 2012 (UTC)
- And would it work if you weren't near one of the poles? 203.27.72.5 (talk) 02:01, 18 August 2012 (UTC)
- Not as well, no. StuRat (talk) 02:06, 18 August 2012 (UTC)
Invisibility
Is there any scientific theory that could potentially cloak objects or is that something that will be confined to sci go & Harry Potter. For example, could destructive interference of visible light be used? — Preceding unsigned comment added by Clover345 (talk • contribs) 16:13, 17 August 2012 (UTC)
- Even if you destroy the light that is reflected by an object, you still would be able to see it, unless you reconstruct the light rays that would be going through the place this object is occupying, if it were not there. Qpl87 (talk) 16:35, 17 August 2012 (UTC)
- Yes, invisibility is theoretically possible. They've even done it in a very limited sense. You can either bend light around an object to hide it, or you can have cameras on one side of the object record the image on that side, and a screen on the other side display that (this only makes it invisible from one POV, however). Or, of course, an object can be made of transparent materials, if that counts. StuRat (talk) 16:39, 17 August 2012 (UTC)
- The articles about the state of the art in invisibility: Transparency and translucency, and camouflage. Qpl87 (talk) 16:47, 17 August 2012 (UTC)
- There was an even better article, but I can't seem to find it for some reason... StuRat (talk) 04:56, 18 August 2012 (UTC)
See Metamaterial cloaking, Adaptiv, Cloak_of_invisibility#Cloaks_of_invisibility_in_science and Active_camouflage#In_research. 203.27.72.5 (talk) 05:38, 18 August 2012 (UTC)
- Invisiblity is quite possible, current research have achieved near perfect invisibility using metamaterials to refract and reflect ambient light around an object. However, it has its limitations - currently, it only works in the microwave range, and on the microscale. IMHO, these are more technological limitations than scientific limitations. Plasmic Physics (talk) 05:47, 18 August 2012 (UTC)
- Meaning "...more technological limitations than theoretical scientific limitations." StuRat (talk) 18:35, 18 August 2012 (UTC)
- Yes, I had a brain-fart at that moment. Plasmic Physics (talk) 23:20, 18 August 2012 (UTC)
- One issue with this approach to invisibility is that you would be effectively blind while under the "invisibility cloak." If all of the light is being moved around you, none of it reaches your eyes. For a Harry Potter style cloak that is implemented technologically you would need either imperfect invisibility or some sort of active solution that can make up for the light that reaches your eyes. 209.131.76.183 (talk) 14:51, 20 August 2012 (UTC)
- Reminds me of the Cone of Silence. StuRat (talk) 05:03, 21 August 2012 (UTC)
- So, poke some eye holes in it. 203.27.72.5 (talk) 20:39, 20 August 2012 (UTC)
- The cloak bends only in a certain wavelength range, ergo it only blocks ina certain range. Plasmic Physics (talk) 23:44, 20 August 2012 (UTC)
- But then you'll see the colours of the outside world wrongly. Double sharp (talk) 10:23, 21 August 2012 (UTC)
Tinned pineapple poisoning?
Please note, this isn't a request for medical advice - I've already 'advised myself' to take it easy, and see a doctor if it doesn't resolve itself soon, though I'm already recovering rapidly. In any case, this is only a hypothesis, and "don't eat it if it tastes off" is hardly medical advice - doh!
Could tinned pineapple in a leaky can ferment sufficiently to cause 'food poisoning like' effects without making the contents look inedible? Having consumed a tin of the stuff on wednesday evening that at the time I thought tasted a little odd, I found myself a few hours later feeling distinctly queasy, and by the following morning I was projectile vomiting spectacularly. Initially I thought that perhaps I was coming down with gastric flu, but given that I'm now almost recovered, this seems unlikely. So, purely hypothetically, could such effects result from a leaky pineapple tin? Could the contents ferment, and would it produce the quantities of alcohol(s) sufficient to bring about the results described? It doesn't strike me as an obvious breeding ground for food-poisoning type bacteria etc. I've found a little on the subject via Google, but it is all rather anecdotal. AndyTheGrump (talk) 16:21, 17 August 2012 (UTC)
- It could certainly cause food poisoning, but not from alcohol. The bacteria consumed and/or their toxins would be the cause. If it tastes or smells "off", don't eat it (it may not look rotten until later). If the can has lost it's seal, don't eat it (the lack of the suction sound of the vacuum breaking when you open it clues you in to this). StuRat (talk) 16:46, 17 August 2012 (UTC)
- I'm not sure whether botulism couldn't grow in tinned pineapple. The USDA says that it is limited to low-acid vegetables , but I see an anecdotal forum report claiming otherwise ... I don't know what I believe. There's no law of nature that says a bacterium can't learn how to resist acid, and doesn't have any strains that can. Obviously other bacteria are possible. But so are food allergies, or an unrelated cold. Wnt (talk) 17:07, 17 August 2012 (UTC)
- I've seen plenty of moldy citrus, so it certainly is possible. I've noticed that it looks quite different on the outside of the moldy fruit, where you get furry bits of white, versus inside, where it tends to just get dark and mushy. Was the pineapple dark and mushy ? StuRat (talk) 17:33, 17 August 2012 (UTC)
- No - it looked fine. AndyTheGrump (talk) 18:00, 17 August 2012 (UTC)
- I once found a jar of apple sauce, looked fine, was unopened, tasted alright, and was free, so I ate it. Later that night I was not feeling well at all - the apple sauce expired 6 years previous... can't always trust your nose/tongue. 65.95.22.16 (talk) 19:44, 17 August 2012 (UTC)
- The OP mentions 'gastric flu', as if it were something distinct from 'food poisoning'; that is not the case. Both terms refer to the same thing: food borne illnesses. Food borne illnesses are almost always a result of infection by microbes (bacteria, viruses, other little beasties) or, more rarely, their metabolites, as with botulism. What I mean by this is that, whether the causative agent came from the tin or from something else entirely, it's virtually a certainty that it was infectious agent and terms like 'flu' vs 'poisoning' are mostly non-starters.
- Now, a hearty bout of norovirus typically lasts a day or so with projectile vomiting as the central attraction. That's much more consistent with what went on than botulism, which presents rather differently (i.e. paralysis). There are plenty of other causative agents, though, so this should not be seen as an attempt at diagnosis.
- Finally, since most foodborne illnesses result from cross-contamination, the visual or olfactory inspection of food is almost worthless for determining whether it's good to eat or not - think of it as a "no only" test; if it looks or smells bad, pitch it, but looking or smelling good means nothing. Matt Deres (talk) 01:56, 22 August 2012 (UTC)
- Vomiting is bad enough, but projectile vomiting is just beyond the pail. :-) StuRat (talk) 02:02, 22 August 2012 (UTC)
Spider identification

What type of spider is this? I haven't been able to find any good information on how you're supposed to measure spiders, but I did include a best-effort photograph of a ruler in this composite picture. I'd say its length sans-legs is roughly 15–20mm. It was found in southern Saskatchewan, if that helps narrow it down.
As a follow-up question, I'm wondering whether my amateur pictures are worth uploading to commons in a less compressed form; although that's probably a help desk question, if anyone has an opinion feel free to voice it. The three shots on the top of my composite picture here are the best ones I got, and I have hi-res un-cropped versions of them, but I have my doubts as to the value of the photographs. BigNate37(T) 18:04, 17 August 2012 (UTC)
- It's an orb-web spider, but that's not narrowing it down that much - perhaps an Araneus of some kind, such as the barn spider. Mikenorton (talk) 18:07, 17 August 2012 (UTC)
- Wow, I actually saw the barn spider article soon after you mentioned orb-web spiders, but I didn't pay it much thought until you mentioned it explicitly. Upon closer examination, it may well be a barn spider, though several of the images at commons:Category:Araneus cavaticus look quite a bit different. Those listed at http://www.iowavoice.com/2009/09/13/barn-spider/ bear a lot more similarity. Thanks for taking a look, Mike. BigNate37(T) 18:38, 17 August 2012 (UTC)
- The markings are unclear but it may also be the female bridge spider (or gray cross spider, see commons:Category:Larinioides sclopetarius), another orb-weaver and notable for being synanthropic (living in human habitations). Also the difference you see in the commons pictures for A. cavaticus is merely sexual dimorphism. Your spider is very likely female, since they're usually the ones which build webs.-- OBSIDIAN†SOUL 00:02, 18 August 2012 (UTC)
- I see. The body proportions and general posture of the limbs of my spider seem to match up better with the barn spider, but the habits fit the bridge spider better: she would hide behind a piece of the sheet metal on the spare tire frame during the daytime, and sat in the centre of her web after dark. BigNate37(T) 00:18, 18 August 2012 (UTC)
- Note though that the size of the abdomen varies depending on if the spider has recently fed or not. The pattern would be a better way to tell. For Larinioides sclopetarius see . For Araneus cavaticus, see . It also looks very similar to the *other* barn spider, Neoscona crucifera, see . The cross orbweaver (Araneus diadematus) is also another possibility, see .
- One way to distinguish the four is that A. cavaticus has small "knobs" or "shoulders" on the front part of their abdomens, kinda resembling the cat-faced spiders (Araneus gemmoides). It also has curved shallow C-shaped or comma-like white patterns on the underside of their abdomens. L. sclopetarius also has elongated comma-shaped to quarter note-like white patterns on the underside but lack the "shoulders". N. crucifera has a broken L white patterns on the underside and also lack the "shoulders". A. diadematus are usually more colorful, have somewhat "squared-off shoulders", and have C-shaped white patterns on the underside as well as distinctive white cross patterns on the upper side of their abdomens.-- OBSIDIAN†SOUL 06:01, 18 August 2012 (UTC)
- It not having been pointed out explicitly, Araneus cavaticus is the Barn Spider, the best known example of which is Charlotte, form Charlotte's Web. μηδείς (talk) 06:30, 18 August 2012 (UTC)
Archery Accuracy
I was once told that elite target archery can be more accurate than target pistol shooting - presumably only at standard competitive archery distances (70m in the Olympics), and presumably when neither sport uses optical aids. Is this true? (] mentions "...the excellent accuracy of modern equipment..." but I cannot find anything that compares the two sports' accuracy. Tom Haythornthwaite 19:34, 17 August 2012 (UTC) — Preceding unsigned comment added by Hayttom (talk • contribs)
- Of course some archer can deliver his arrows more accurately than some pistol shooter. But I would imagine that for someone with no prior experience with either, accurately shooting the pistol would be easier than shooting a bow and arrow. I shoot mainly rifles myself, but I do shoot pistol too. I've used a bow and arrow maybe twice ever, and I remember it being very difficult. Within the handgun dispilines there's also a wide array of different classes that are not all as easy to shoot accurately as each other, and they don't all have the same intrinsic accuracy. A black powder pistol that fires a lead ball is intrinsically less accurate than a revolver firing a bullet due to the aerodynamic aspects. A double action revolver is harder to shoot accurately than a semi automatic pistol because as the trigger is pulled in the revolver it cocks the hammer which causes the gun to want to move due to the hammer's inertia. You don't have that problem in the semi-auto because the firing pin was already cocked by the recoil from the previous round, or for the first round you would have cocked it manually. 203.27.72.5 (talk) 20:30, 17 August 2012 (UTC)
- It's good to get the perspective of an actual gun shooter. I suppose I really want to know about the relative accuracy of top competitive archers and shooters; if they were aiming at the same target under the same conditions (within competitive archery range) who would win? Tom Haythornthwaite 20:37, 17 August 2012 (UTC) — Preceding unsigned comment added by Hayttom (talk • contribs)
- Well, I thought I would just compare the world record in archery against the world record in pistol shooting, but unfortunately the Olympic pistol event is over 50m and the Olympic archery event is over 70m, so it's hard to compare them. 203.27.72.5 (talk) 21:45, 17 August 2012 (UTC)
- I teach some basic archery to kids and have been on courses where they talk about competition shooting. The standard competition archery "face" (archers have their own jargon) for FITA events at 50m is 80cm (32 in?) with the "gold" (which scores 9 or 10) being 155mm (6 in) across. However the 50m pistol target is 50cm (20in) with the centre being only 50mm (1.9in) in size.. Good archers do get pretty tight groups at 50 or 70m, but can't get too tight as the first arrows tend to obstruct the path of the later arrivals (although you can move sideways along the shooting line to try to minimize this problem). So based on the size of the target, I would say that the expectation is that pistols are going to be more accurate than bows. Alansplodge (talk) 00:02, 18 August 2012 (UTC)
Thank you, Alansplodge. Tom Haythornthwaite 05:27, 18 August 2012 (UTC)
- Hmm, I think all the posts here have missed the point! The anecdote seems to work precisely because it IS counter intuitive! So you'd EXPECT the regular targets to be smaller for pistols, because they're for regular people; but the question is not about regular archers, but about ELITE archers.. I actually do think it's kind of a pointless anecdote that you probably can't really prove anyway. Come to think of it, perhaps this is a saying from the olden days when flint lock pistols 1st became popular. Only rich people could afford them and would have thought they were the bees knees, but someone who still shot with a bow could reply that elite target archery can be more accurate than target pistol shooting. Vespine (talk) 23:45, 19 August 2012 (UTC)
- The dimensions of the targets are specified in the respective sport's regulations - the "bullseye" in archery is 6 inches wide, the pistol target "bullseye" is less than 2 inches in diameter. It has nothing to do with "regular" and "elite" archers or pistol shooters, the target regulations remain the same. You seem to have misinterpreted the word "regular" to mean "ordinary" while the actual meaning is "according to the regulations". Roger (talk) 21:34, 21 August 2012 (UTC)
Adrenaline rush
How do adrenaline rushes counter stress. For example, many people claim activities such as roller coasters or parachuting help relieve stress? Aren't prolonged adrenaline rushes dangerous as it leads to elevated heart rates which means blood is pumped less efficienty around the body or is that only in people with existing heart conditions? Note this is not medical advice and I'm not asking for an answer which contains advice. Clover345 (talk) 20:52, 17 August 2012 (UTC)
- Short term it can be good, just like in exercise, as it gets your heart pumping, etc. Also, faced with a real or simulated life-and-death situation makes things we normally stress about seem insignificant, at least for a while. StuRat (talk) 20:55, 17 August 2012 (UTC)
- thanks but what is the actual reason these activities relieve stress without being harmful? Clover345 (talk) 21:38, 17 August 2012 (UTC)
- I don't think that adrenaline rushes per se counter stress. For example, suppose you are locked in a room with a hungry bear. Each time the bear looks at you, you will get an adrenaline rush. They don't counter your stress, though, they accumulate to increase it. What counters stress in the examples you mentioned is more likely the pleasure associated with the experiences, manifesting itself as a dopamine rush. Looie496 (talk) 01:09, 18 August 2012 (UTC)
- is there a source for where this notion that they relieve stress is coming from? I have sought and not found. μηδείς (talk) 04:44, 18 August 2012 (UTC)
- I don't think that adrenaline rushes per se counter stress. For example, suppose you are locked in a room with a hungry bear. Each time the bear looks at you, you will get an adrenaline rush. They don't counter your stress, though, they accumulate to increase it. What counters stress in the examples you mentioned is more likely the pleasure associated with the experiences, manifesting itself as a dopamine rush. Looie496 (talk) 01:09, 18 August 2012 (UTC)
- thanks but what is the actual reason these activities relieve stress without being harmful? Clover345 (talk) 21:38, 17 August 2012 (UTC)
Time Dilation Questions
I have been directed here by DVdm with regards to a comment that I made on the article Time Dilation. This is located at:
http://en.wikipedia.org/Time_dilation
The section of the article entitled “Simple inference of time dilation due to relative velocity” concludes by stating that Δt’ = γΔt where γ is the Lorentz factor.
I wrongly assumed that this equation was in error because I did not take the setup properly into account. (Out of context it reads that moving clocks run fast).
However, when I analyzed the proof in that section, I stated that the bottom drawing could not be Euclidean because there was relative motion between the rest observer O and the moving observer whom I will designate as O’.
DVdm stated that the analysis “…in this case is used in the context of a spatial vector triangle in the Euclidean geometry of a 2-dim strictly spatial schematic drawing”.
I do not understand his comment.
In my mind the following are problems with that statement:
1) If the problem is strictly spatial then Δt and or Δt’ are 0 and the conclusion that Δt’ = γΔt is trivial.
2) If one looks at the problem from the point of view of observer O (and ignores the fact that O’ is moving), then the conclusion that Δt’ = γΔt will not be valid because as DVdm has stated the conclusion is valid from the point of view of O’.
3) If one looks at this from the point of view of O’ then the mirrors are moving and I can not envision a way to see this as a Euclidean space. This in turn means that the Pythagorean Theorem is not valid. But the Pythagorean Theorem is critical to the proof.
I would appreciate any guidance you can give me on this matter.
Thanks. Emagnus3 (talk) 21:33, 17 August 2012 (UTC)
- 3) The validity of the Pythagorean theorem in this context is an assumption of the argument. To say that "the speed of light is constant" is to say that is a constant (namely c) for straight-line motion, where x, y, z, t are coordinates defined by some inertial reference frame (a network of metersticks and Einstein-synchronized clocks).
- 2) Δt’ = γΔt is valid independent of reference frame, because all relevant reference frames are mentioned in the definitions of the variables. Δt and Δt' are elapsed coordinate times of frames O and O' respectively, and γ depends on the relative velocity of O and O'.
- The argument is valid. It's fine to dislike it, though. I dislike it. It's superficially convincing because it exploits Euclidean/Newtonian intuitions, but special relativity shows that you shouldn't trust those intuitions. It's usually a bad idea to separate x, y, z from t in special relativity and pretend that you live in a quasi-Newtonian world with weird non-Newtonian "effects". -- BenRG (talk) 01:04, 18 August 2012 (UTC)
- For what it's worth, an argument I like better starts from the assumption of "relativity of redshifts"—roughly that if two people are moving inertially away from each other in outer space, and they point identical radar speed guns at each other, both guns will report the same speed. See k-calculus. -- BenRG (talk) 01:27, 18 August 2012 (UTC)
- Spacetime is still flat (ie it's a Minkowski space) in special relativity, so the Pythagorean theorem is valid. It's only when you start fiddling with the metric tensor in general relativity that you need to worry about how to take the norm. --99.227.95.108 (talk) 04:42, 18 August 2012 (UTC)
- Well, the Pythagorean theorem is valid on a Euclidean subspace of Minkowski space, anyway. The comparable theorem that holds on the entire Minkowski space is that if gαβUV=0, then gαβ(U-V)(U-V) = gαβUU + gμξVV, where g is the Minkowski metric, instead of a Euclidean metric as would be needed to make those equations be an expression of the normal Pythagorean theorem. Red Act (talk) 23:36, 18 August 2012 (UTC)
- You do indeed need more assumptions than what is explicitly stated in that article section in order to conclude that it's reasonable to use the Pythagorean theorem in that section, and there's more than one way of specifying exactly what those postulates are; see Special relativity#Postulates.
- Part of what is indirectly being assumed from the unstated starting set of postulates is that there are multiple ways of decomposing spacetime into a product space of a one-dimensional "time" dimension, and a three-dimensional Euclidean space. Choosing that decomposition is part of what's being done when you choose an inertial frame of reference.
- The bottom drawing in that article section is purely spatial in the O′ frame of reference, because it consists of a projection of the events involved along O′ 's time dimension onto a plane in O′ 's Euclidean space. The bottom drawing would not be purely spatial in the O frame of reference.
- What's being called D in the bottom drawing and the equations should really instead be called D′, to make it clear that it's a distance measured in the primed frame. The L in the bottom diagram should probably also be labeled L′, with an accompanying argument based on the postulates as to why L′=L. Red Act (talk) 23:07, 18 August 2012 (UTC)
Microwave plastic ring
My new microwave, like all others that I have seen so far, has a plastic ring below the glass plate. This is a combination microwave oven and grill and I don't know if its secure to use the grill (400 F) with the ring inside. The manual neither says it's possible, nor that it's not. Is it safe to use it? Qpl87 (talk) 22:20, 17 August 2012 (UTC)
- First, knowing the model of microwave might help. Second, keep in mind that accepting a wrong answer on this might result in your microwave bursting into flame. You may still want to contact the manufacturer, especially if no one can give you a referenced answer. Someguy1221 (talk) 22:40, 17 August 2012 (UTC)
- It's difficult to say as it depends greatly on exactly what the composition of the ring is. Perhaps it's actually glass-ceramic like white casserole dishes? BigNate37(T) 23:02, 17 August 2012 (UTC)
- I'd play it safe and take the ring out. Does it even try to rotate while in grill mode ? StuRat (talk) 02:01, 18 August 2012 (UTC)
- I have seen combination-Microwave ovens that rotate a grill in convection mode that sits atop the glass plate, and whose instructions specifically state to allow the grill to rotate. The design of the grill may make it obvious what was intended by the manufacturer. Someguy1221 (talk) 02:57, 18 August 2012 (UTC)
- I don't have a source, but I do have a microwave, and the plastic ring is of heat-resistant plastic, and it only touches the glass plate at three very small rolling points that will not conduct enough heat to melt the plastic even if I run the oven 20 minutes, which I never do. μηδείς (talk) 06:26, 18 August 2012 (UTC)
- Right, and that's why they don't melt in a normal microwave, but a 400° F microwave/grill is a different story. Presumably the plastic will eventually heat to 400 degrees to match the air temperature. The question is whether it can take that heat. StuRat (talk) 06:31, 18 August 2012 (UTC)
- Well, contact the m'f'er is still the only valid response. μηδείς (talk) 06:36, 18 August 2012 (UTC)
- Right, and that's why they don't melt in a normal microwave, but a 400° F microwave/grill is a different story. Presumably the plastic will eventually heat to 400 degrees to match the air temperature. The question is whether it can take that heat. StuRat (talk) 06:31, 18 August 2012 (UTC)
- If the manual doesn't say to remove the ring, it's probably fine. Manufacturers tend to over-warn users about things that may damage the equipment. For reference, note that the melting point of teflon is 620°F. Just because the ring is plastic, doesn't mean that it will melt at 400°F.--Srleffler (talk) 17:04, 20 August 2012 (UTC)
August 18
Who is this beetle?


What is this lovely beetle? I found it on my front porch today, in Kernville, California. It's about an inch and a half long. --jpgordon 01:13, 18 August 2012 (UTC)
- Looks like a potato beetle with inverted colors. Some variation, perhaps ? StuRat (talk) 01:58, 18 August 2012 (UTC)
- It looks like a female ten-lined June beetle. Although its difficult to see how large its antennae are in the photos, it does not appear to have the large distinctive antennae of the males of this species. -Modocc (talk) 03:09, 18 August 2012 (UTC)
- Yeah female. But it could be any of three easily confused and sympatric species of Polyphylla though - Polyphylla decemlineata, Polyphylla nigra, or Polyphylla crinita. There's a species key here, but different sources offer different contradictory diagnostics. -- OBSIDIAN†SOUL 06:59, 18 August 2012 (UTC)
- Thanks! And indeed, I forgot to mention that this lovely beetle was "hissing" as described in the article. June beetle indeedle! --jpgordon 15:43, 18 August 2012 (UTC)
- That is a mint humbug. 2.97.21.248 (talk) 02:32, 22 August 2012 (UTC)
+9 oxidation state
Can any element take a +9 oxidation state? I heard somewhere on-wiki (now forgotten) that IrO4 would afford the best chance for +9. I also read that meitnerium may be capable of it.--Jasper Deng (talk) 02:49, 18 August 2012 (UTC)
- Apparently spectra of Ir(IX) have been observed. 203.27.72.5 (talk) 04:13, 18 August 2012 (UTC)
The wikipedia article that you read it in was most likely Iridium#Compounds where it says, "it was reported in 2009 that iridium(VIII) oxide (IrO4) was prepared under matrix isolation conditions (6 K in Ar) by UV irradiation of an iridium-peroxo complex.". 203.27.72.5 (talk) 04:16, 18 August 2012 (UTC)Sorry, that's not the cation you're talking about. 203.27.72.5 (talk) 04:19, 18 August 2012 (UTC)- (edit conflict)But that is in an excited state, and therefore technically doesn't count here.--Jasper Deng (talk) 04:33, 18 August 2012 (UTC)
- This might be where you read both of those things . 203.27.72.5 (talk) 04:32, 18 August 2012 (UTC)
- I read that one on-wiki.--Jasper Deng (talk) 04:33, 18 August 2012 (UTC)
- Well that could have been meitnerium, which cites the article I did when it says, "The oxidation state +9 might also be possible for meitnerium in ". 203.27.72.5 (talk) 05:00, 18 August 2012 (UTC)
- I specifically recall the iridium-based cation.--Jasper Deng (talk) 05:04, 18 August 2012 (UTC)
- The paper W203 cites was also mentioned in two previous ref-desk discussions on this topic. DMacks (talk) 17:46, 18 August 2012 (UTC)
- . (I've also seen it before on WP, but, annoyingly, can't remember where right now.) Double sharp (talk) 12:53, 19 August 2012 (UTC)
- Talk:Iron#Fe(VIII)? DMacks (talk) 14:59, 19 August 2012 (UTC)
- Am I not too late to show up with another surprise? (There is no fundamental reason why an OS above +8 couldn't exist in a non-main block element. It's all about energy. Think of HgF4. It is very unstable, but existent. (although it also breaks a school rule, which is, in this case, that a truly (no Madelung exceptions) finished shell other than ns2 may not be broken down). Or Xe compounds (even though they don't break the octet rule, but again you can't correctly draw it with school-style Lewis dots). Even the masses before and after a chemical reactions don't match (E=mc2 :P). Note that none of first four periods elements can step over +7. For no fundamental reason -- just iron doesn't want to form eight bonds. Plus consider the SHE with several shells open at a time (they are complicated). The main problem, aside that the elements don't want to lose electrons (although they defend-- highest OS compounds are never ionic, so that the electrons are not completely away), is that you have to squeeze many fluorides around a single atom, it's hard. Using oxides (two bonds, twice the OS) is a good approach, but they may let you down and bond to themselves rather than the metal (like chromium pentoxide, which is actually "oxide diperoxide"). Sorry if I'm duplicating anything, but I want to make a more-or-less balanced reply (note I'm not a chemist, nor plan to ever be one, so if you're seriously into the idea, find one specializing one the stuff instead))--R8R Gtrs (talk) 22:48, 19 August 2012 (UTC)
- How about Americium, is there potential? Plasmic Physics (talk) 02:40, 20 August 2012 (UTC)
- Well, according to Americium for Am → Am the potential is 2.08V, but for Am → Am I have no idea. 203.27.72.5 (talk) 04:57, 20 August 2012 (UTC)
- A ha, ha, and ha.
- Addendum: ha. Plasmic Physics (talk) 10:27, 20 August 2012 (UTC)
- Some speculation on high oxidation states on a forum – Cs(III), Fr(III), Ir(IX), Am(IX). R8R Gtrs also found this (speculation on high oxidation states of the period-6 transition metals, posted on Misplaced Pages talk:WikiProject Elements/Archive 11#Highest oxidation states of elements osmium through mercury) last year. Double sharp (talk) 10:02, 20 August 2012 (UTC)
- Well, according to Americium for Am → Am the potential is 2.08V, but for Am → Am I have no idea. 203.27.72.5 (talk) 04:57, 20 August 2012 (UTC)
- How about Americium, is there potential? Plasmic Physics (talk) 02:40, 20 August 2012 (UTC)
- Am I not too late to show up with another surprise? (There is no fundamental reason why an OS above +8 couldn't exist in a non-main block element. It's all about energy. Think of HgF4. It is very unstable, but existent. (although it also breaks a school rule, which is, in this case, that a truly (no Madelung exceptions) finished shell other than ns2 may not be broken down). Or Xe compounds (even though they don't break the octet rule, but again you can't correctly draw it with school-style Lewis dots). Even the masses before and after a chemical reactions don't match (E=mc2 :P). Note that none of first four periods elements can step over +7. For no fundamental reason -- just iron doesn't want to form eight bonds. Plus consider the SHE with several shells open at a time (they are complicated). The main problem, aside that the elements don't want to lose electrons (although they defend-- highest OS compounds are never ionic, so that the electrons are not completely away), is that you have to squeeze many fluorides around a single atom, it's hard. Using oxides (two bonds, twice the OS) is a good approach, but they may let you down and bond to themselves rather than the metal (like chromium pentoxide, which is actually "oxide diperoxide"). Sorry if I'm duplicating anything, but I want to make a more-or-less balanced reply (note I'm not a chemist, nor plan to ever be one, so if you're seriously into the idea, find one specializing one the stuff instead))--R8R Gtrs (talk) 22:48, 19 August 2012 (UTC)
- Talk:Iron#Fe(VIII)? DMacks (talk) 14:59, 19 August 2012 (UTC)
- . (I've also seen it before on WP, but, annoyingly, can't remember where right now.) Double sharp (talk) 12:53, 19 August 2012 (UTC)
- Well that could have been meitnerium, which cites the article I did when it says, "The oxidation state +9 might also be possible for meitnerium in ". 203.27.72.5 (talk) 05:00, 18 August 2012 (UTC)
- I read that one on-wiki.--Jasper Deng (talk) 04:33, 18 August 2012 (UTC)
Specialization related to identification of animal sounds
Is there any specilization dealing with identification of sounds by animals (in the context of animal husbandry). Who are the well known figures in this speciality area Would appreciate any help183.83.244.183 (talk) 07:57, 18 August 2012 (UTC)vsmurthy
- There's bioacoustics (and the related ecoacoustics). Identification by sound is a large part of the work of bioacousticians (especially among ornithologists). I don't know of any subfields of those specializing in domesticated animals though, as the usual subjects are birds, marine mammals, fish, anurans, and insects. There's also zoosemiotics, the study of animal communication in general.-- OBSIDIAN†SOUL 10:18, 18 August 2012 (UTC)
Hi, I need help with this endocrionological equation
There is a link for a picture of it.
I have understood the more basic equations to it, but i have lost that one, and i have tried some times... it appears in chapter 1 of Greenspan's endocrinology textbook. please explain it to me, i must understand it. many thanks. 79.181.146.146 (talk) 09:44, 18 August 2012 (UTC)
- To save others the trouble, here is the equation in question:
- Given the (meagre) context you've offered, I would presume that H is hormone, R is receptor, and is their dissociation constant.
- You can multiply both sides by to get a somewhat simpler expression. Then we know that, by definition:
- The equation then becomes . This seems pretty obvious (receptor concentration equals original/total concentration minus what is bound).
- Regarding what the original equation "means" - that would depend on what is known about a system (e.g. which variables are already known or measurable) and what is desired; however, you could make some guesses about what each term in the original expression means based on what I've said. BTW, is this homework? -- Scray (talk) 19:51, 18 August 2012 (UTC)
- Ah, one more thing about how that equation might be useful. You might want to have a look at Scatchard plot, and consider that is really the ratio of bound to unbound hormone, and is the bound fraction. Now, if you compare your original equation to y = mx + b, can you see what you could learn from a Scatchard plot? -- Scray (talk) 20:02, 18 August 2012 (UTC)
- Hello Scary, so much thanks for your deatiled explaination. it's not homework !. i just came across it at the library.. i have yet to do that comparison. first i need to make sure i understood you right: that, R is R0---which is the sum of the dissociation between H+R..?
- btw, why should we divide : shouldn't it be the opposite?: , ie: equals the HR complex divided by H+R?. thanks !. 79.177.146.177 (talk) 21:02, 18 August 2012 (UTC)
- I did not say that "R is R0", nor that it is "the sum of the dissociation...". I meant to imply that is unbound receptor, and is the total receptor concentration, i.e. (but I am not the one who was looking at the book - I am making inferences). For an explanation of the equation, have a look at Dissociation constant. -- Scray (talk) 21:14, 18 August 2012 (UTC)
What is John Money's intention?
In the definition of the evolutionary term "Phylism" (page 85) ? — Preceding unsigned comment added by 109.65.177.63 (talk) 10:24, 18 August 2012 (UTC)
Black hole = Center of mass?
I am just throwing out this intuitive idea , how about this:
Black holes are formed at the point where the center of mass would be for massive objects such as galaxy's and globular clusters.
The mass of the object causes the distortion of space/time at the point where the center of mass would be and hence a black hole (or perhaps only super massive black holes).
92.23.128.134 (talk) 11:14, 18 August 2012 (UTC)a-uk
- The centre of mass may be a future black hole, but a black hole event horizon will not appear around the space that is empty. He potential energy will be lower at one of your masses. Graeme Bartlett (talk) 12:25, 18 August 2012 (UTC)
- "He potential energy" = the energy only released by a male couch potato when his team scores a goal on the TV. StuRat (talk) 18:31, 18 August 2012 (UTC)
- You may find the shell theorem interesting. As it shows, there isn't necessarily any gravitational field at the centre of an object. Black holes form because of a concentration of matter at a point, not because of matter distant from that point but centred on it. --Tango (talk) 13:54, 18 August 2012 (UTC)
- According to the M-sigma relation there is a close correlation between the central bulge of galaxies and the black holes that form. An idea mentioned there is that the hole predates much of star formation, triggered by a "collapse of the central bulge". The hole is indeed formed by a distortion of spacetime, i.e. gravity. Wnt (talk) 14:19, 19 August 2012 (UTC)
Plant identification
Hi! Can anyone identify the plant in these photos? In the space of a couple months, it's taken over almost our entire garden. Wide Mid Close - Thanks very much! 77.97.198.48 (talk) 19:13, 18 August 2012 (UTC)
- Possibly relevant: location is Scotland. 77.97.198.48 (talk) 19:18, 18 August 2012 (UTC)
- It looks to me as if it may quite possibly be Himalayan Balsam (Impatiens glandulifera), a rather troublesome invasive species now found in parts of Scotland If this is indeed the case, I wish you luck in getting rid of it. You may be occupied for some time. AndyTheGrump (talk) 19:57, 18 August 2012 (UTC)
- That looks like a strong contender, thank you - although the photos I've found online seem to show serrated leaves, which are absent (certainly that I noticed, I will check again in the light tomorrow) from ours. Would that rule it out, or could it simply be a smooth-leafed variation? Thanks again. 77.97.198.48 (talk) 21:18, 18 August 2012 (UTC)
- Actually, after a little more digging, we've come across Himalayan Honeysuckle which looks exactly like what we have, so I'm going to mark this one resolved. Thanks for setting us on the right path! 77.97.198.48 (talk) 21:30, 18 August 2012 (UTC)
- That may not necessarily be good news either - Himalayan Honeysuckle is yet another invasive species apparently. It might be worth contacting the Scottish Environment Protection Agency, via one of their local offices (see here ) if you are still at all in doubt about the identification. I'm sure they'll be only too familiar with abominable Himalayan
beastiesinvaders, and with other possibilities and will be able to confirm one way or another. If it is the Balsam or the Honeysuckle, your only consolation is that at least you haven't been infested with Giant Hogweed (Heracleum mantegazzianum), another invader that adds phototoxicity to the long list of reasons not to like it. Sadly, along with Himalayan Balsam, and Japanese Knotweed (which can swallow locomotives whole, as our article illustrates ), it was originally introduced to the British Isles deliberately for ornamental reasons. Not a good idea... AndyTheGrump (talk) 21:56, 18 August 2012 (UTC)
), it was originally introduced to the British Isles deliberately for ornamental reasons. Not a good idea... AndyTheGrump (talk) 21:56, 18 August 2012 (UTC)
- That may not necessarily be good news either - Himalayan Honeysuckle is yet another invasive species apparently. It might be worth contacting the Scottish Environment Protection Agency, via one of their local offices (see here ) if you are still at all in doubt about the identification. I'm sure they'll be only too familiar with abominable Himalayan
- It's hard to see how a plant awarded the AGM by the Royal Horticultural Society could be regarded as a garden pest! It spreads easily because the berries get eaten by birds and redistributed. All you need to do is pull it up if you don't like it. --TammyMoet (talk) 09:37, 19 August 2012 (UTC)
- Opinion in the UK seems to be somewhat divided regarding the Himalayan Honeysuckle. In other parts of the world, it is unquestionably regarded as a serious invasive weed. New Zealand seems to have the worst problem, but the Australians don't appear to appreciate it either , and I doubt that de-weeding National Parks and mountain ranges by hand is practical. AndyTheGrump (talk) 18:54, 19 August 2012 (UTC)
- If it's anything like Japanese knotweed, an invasive garden plant that's gone on the rampage in many parts of Europe, it's not quite as simple as "pull it up if you don't like it". I decided to expunge a clump of knotweed about 7 years ago. I spent a half-day digging it out and burning every fragment of the roots and the knotty rhizomes that I could find (moving them anywhere else is a criminal offence). I am still pulling up new shoots of the wretched stuff today (well Friday actually). Alansplodge (talk) 22:19, 19 August 2012 (UTC)
- Ok, apologies Tammy, apparently you can "just pull it up" as long as you don't disturb the seed pods - see AndyTheGrump's link above. Alansplodge (talk) 17:59, 20 August 2012 (UTC)
- If it's anything like Japanese knotweed, an invasive garden plant that's gone on the rampage in many parts of Europe, it's not quite as simple as "pull it up if you don't like it". I decided to expunge a clump of knotweed about 7 years ago. I spent a half-day digging it out and burning every fragment of the roots and the knotty rhizomes that I could find (moving them anywhere else is a criminal offence). I am still pulling up new shoots of the wretched stuff today (well Friday actually). Alansplodge (talk) 22:19, 19 August 2012 (UTC)
Why the climate is not completely cyclic?
Hi,
Why the climate is not completely cyclic? I mean, why in one day in different years, there are different temperatures? Exx8 (talk) 21:52, 18 August 2012 (UTC)
- The Earth's climate is the result of extremely complex interactions - the planet's annual trip around the sun certainly plays a big role, but there are just so many other factors involved that it just isn't reasonable to expect the temperature of any given point to be consistent from one year to the next. 66.87.127.70 (talk) 23:11, 18 August 2012 (UTC)
- It's all the fault of those damned butterflies. Clarityfiend (talk) 23:45, 18 August 2012 (UTC)
- I cannot resist sharing this comic. --Mr.98 (talk) 00:26, 19 August 2012 (UTC)
- Yes, tiny random events, like the proverbial flapping of butterfly wings, cause larger changes, eventually resulting in global changes in weather patterns. There are also other cycles, like the approximately 5-year el Nino cycle and the 11.2 year sunspot cycle which can affect the weather. StuRat (talk) 23:48, 18 August 2012 (UTC)
- It's all the fault of those damned butterflies. Clarityfiend (talk) 23:45, 18 August 2012 (UTC)
- Do tiny events like that really cause global changes in weather patterns? One time events I can see, but changing the pattern of events seems a bit of stretch to me. I could be mistaken. 203.27.72.5 (talk) 00:26, 19 August 2012 (UTC)
You may be interested in Milankovitch cycles. In brief, even if the year were exactly 365 days long, the Earth is not in the same orientation every January 1st. Throw in small year-to-year variations in Solar brightness, the apparent chaotic nature of weather, weather effects of volcanoes, industry, etc... What you're left with is no reason to suspect that climate should be perfectly cyclical. Someguy1221 (talk) 01:42, 19 August 2012 (UTC)
- It is; the cycle is simply longer, on monkeys and typewriters scale. μηδείς (talk) 01:57, 19 August 2012 (UTC)
In addition to the good reasons mentioned above, climate is not even an abiotic phenomenon. Biogeochemistry plays a large role in determining how Earth's climate has changed across long time scales. SemanticMantis (talk) 02:30, 19 August 2012 (UTC)
August 19
Pressure increase on the ground due to an overflying plane
A plane of mass m flies at some altitude h (say h = 10 km) above the ground. The force of gravity acting on the plane is ultimately transferred to the ground, so the pressure at the ground will increase due to an overflying plane and this obviously depends on the position on the ground relative to the plane. The problem is then to compute this pressure increase from first principles. You can only simplify things by assuming that the plane is very far away from the ground. Count Iblis (talk) 03:40, 19 August 2012 (UTC)
- Sounds like homework. The problem is not knowing how the pressure is distributed. If forced to guess, I'd go with a 45 degree cone with equal pressure applied to all the ground under it. Has anyone actually studied this to determine the true force distribution ? StuRat (talk) 04:04, 19 August 2012 (UTC)
- The pressure quickly spreads out spherically. The mean free path of an air molecule, which moves at 1,000kmph at STP is 10 cm. This conical notion seems to imply you are forgetting air is a gas. μηδείς (talk) 04:12, 19 August 2012 (UTC)
- Spherically ? Do you mean hemispherically ? Certainly a plane doesn't increase the pressure above it. StuRat (talk) 04:15, 19 August 2012 (UTC)
- Yes, it does, there is nothing to constrain it. Consider a sonic boom. It doesn't just travel downward or in one direction. When you blow up a balloon, it does not expand only on the surface opposite your mouth. You are imagining a gas as if it were a pile of sand with a critical angle of repose. The atmosphere consists of particles bouncing off each other in random directions at 1,000 kmph at STP. Any change in pressure (and actually there is no real net change in pressure except the from the heat generated by the flight, since the difference in pressure above and below the wing is quickly neutralized as the plane passes) is rapidly transmitted in every direction. μηδείς (talk) 04:27, 19 August 2012 (UTC)
- It can be useful to think of the atmosphere as an ocean of air. Airplanes, especially those flying at high speeds create a "wake" in the air just as boats do on a pond. And if another traveling object hits that wake, it can cause it to "bounce" in the unstable substance. ←Baseball Bugs carrots→ 05:00, 19 August 2012 (UTC)
- Yes, but the wake (of turbulence, not pressure) soon dissipates, and is not a cone of pressure beneath the airplane. μηδείς (talk) 05:06, 19 August 2012 (UTC)
- I think I see the difference. However, I've been on planes when we got bounced around a bit, and the pilot said we were in the wake of another plane. ←Baseball Bugs carrots→ 05:52, 19 August 2012 (UTC)
- Yes, wake turbulence is a very real thing, but it is different to the equal and opposite reaction to the lift, which is what the OP is asking about. --Tango (talk) 10:45, 19 August 2012 (UTC)
- Fascinating question with very real implications, and very difficult physics to contemplate. Every time I land my little Citabria at San Jose International Airport, the tower warns me to watch out for vortices and wake turbulence from the big 737s on every single approach - even if I'm the only aircraft around - just in case the vortex from the last jet is still "floating around" in the air. The textbook-solution (Chapter 7 section 3 of the AIM) has some nice cartoon diagrams of persistent wake and vortex effect. 7-3-4 discusses sink-rate of persistent vortices - in other words, how long until the wake "hits" the ground. So, that's how to solve it not from first principles, but in a way that provides pilots with useful information.
- From first-principles, the assumption is invalid: air is compressible, so the dynamic pressure that creates lift near the aircraft need not ever propagate to the ground. It could aerodynamically convert to another form of energy, such as compression-heating. After the aircraft passes, it could return to its original state. Energy is conserved, and momentum is conserved, but there is no law of conservation of pressure, temperature, or force. In an ideal, perfect, skin-frictionless system with perfect laminar flow, the air should flow smoothly around the aircraft, and no energy need be lost to propagating a pressure wave away from the aircraft surface.
- In the vortex wake turbulence case, the air flow is not propagating as a pressure wave - it is a turbulent, convecting effect. For comparison, the sound of the engines is propagating as a pressure wave, at much higher frequencies. It would be interesting to compare total energy or power in each propagating mode. I suspect more energy is propagating as low-frequency convecting turbulence, rather than as an acoustic pressure wave. NASA has studied wake turbulence and the vortex effect very thoroughly - there's a classic photo that is now a featured picture - aircraft vortex effect. To compute these sorts of things from first principles, as the OP requested, is nearly impossible: but that's how we derive the basic laws of air flow such as Navier Stokes. I think the problem is the assumption that the dynamic air motion can be expressed simply: but in an enormous mass of thermally-inhomogeneous, convecting air, simple pressure-waves are so unphysical as to be misleading. Suffice to say, the aircraft wing loading is sustained by the dynamic pressure of the air close to the aircraft; and that air is supported by the dynamic pressure of the air near it, ... and ad infinitum, all air that ever contacts any other air exchanges energy and momentum through complicated aerodynamic processes. The speed of propagation of each effect is different: there is an effective wave speed for pressure, for shear/vortex propagation, for thermal conduction, and so on. Each process conveys some of the energy, and some of that energy translates into transient, dynamic pressure. Because air is compressible, it's meaningless to talk about only the pressure wave propagating. You must consider the other aerodynamic effects. Nimur (talk) 16:00, 19 August 2012 (UTC)
- Yes, wake turbulence is a very real thing, but it is different to the equal and opposite reaction to the lift, which is what the OP is asking about. --Tango (talk) 10:45, 19 August 2012 (UTC)
- I think I see the difference. However, I've been on planes when we got bounced around a bit, and the pilot said we were in the wake of another plane. ←Baseball Bugs carrots→ 05:52, 19 August 2012 (UTC)
- Yes, but the wake (of turbulence, not pressure) soon dissipates, and is not a cone of pressure beneath the airplane. μηδείς (talk) 05:06, 19 August 2012 (UTC)
- It can be useful to think of the atmosphere as an ocean of air. Airplanes, especially those flying at high speeds create a "wake" in the air just as boats do on a pond. And if another traveling object hits that wake, it can cause it to "bounce" in the unstable substance. ←Baseball Bugs carrots→ 05:00, 19 August 2012 (UTC)
- Pressure always acts in all directions. That's the main thing that distinguishes between it and other forces. Hold you hand out in front of you, flat to the ground. The mass of the column of air above it is about 8kg and the weight of that is pressing down on your hand. That's the air pressure. You can hold your hand up easily, though, because there is an equal pressure on the bottom of your hand and the forces cancel out. While the pressure on the top of your hand can be thought of as the weight of the air above it, the pressure of the bottom is a little more difficult to understand - it's caused by the rest of the atmosphere pressing down on the air next to your hand, which pushes on the air under your hand, which pushes up on your hand. --Tango (talk) 10:45, 19 August 2012 (UTC)
- Yes, it does, there is nothing to constrain it. Consider a sonic boom. It doesn't just travel downward or in one direction. When you blow up a balloon, it does not expand only on the surface opposite your mouth. You are imagining a gas as if it were a pile of sand with a critical angle of repose. The atmosphere consists of particles bouncing off each other in random directions at 1,000 kmph at STP. Any change in pressure (and actually there is no real net change in pressure except the from the heat generated by the flight, since the difference in pressure above and below the wing is quickly neutralized as the plane passes) is rapidly transmitted in every direction. μηδείς (talk) 04:27, 19 August 2012 (UTC)
- The problem with the "cone" model is that the force won't immediately be transmitted to the ground, but will be transmitted as some sort of slow pressure wave. The problem is a bit similar to blowing briefly at the far wall of a room - where will the breath impact? - except that because the plane has since moved the area "supporting" it will be far behind it when it reaches the ground. A difficult and interesting question. Wnt (talk) 14:27, 19 August 2012 (UTC)
- As pointed out above, a plane's downwash spreads outward and dissipates rapidly. When helicopters and planes fly low enough, this downwash interacts with the ground (see the article on ground effect (aircraft) for instance). With helicopters their downwash can create vortex rings of air that are dangerous, because the air mass in which the copter is in starts to descend rapidly. Air vortex cannons can create these vortex rings which can hit targets such as the ground, and these cannons can be as simple as thumping the bottom of an empty one-gallon milk jug. Modocc (talk) 15:19, 19 August 2012 (UTC)
- Spreads outward, yes; dissipates, somewhat, at least in terms of energy - but one thing it cannot do is dispose of its downward momentum, except by impacting something to transfer it to. Wnt (talk) 15:43, 19 August 2012 (UTC)
- The downward momentum is quickly transferred and scattered amongst a large number of molecules though, adding a nearly insignificant downward movement to the air in a much larger surrounding atmosphere. The entire Earth moves too, eventually, but that happens every time I get out of bed. Also, the energy of the downwash is still dissipated, thus when it has no more energy, it no longer transfers momentum to the atmosphere. -Modocc (talk) 16:20, 19 August 2012 (UTC)
- This is related to that old thought experiment/joke of getting a van full of birds over a bridge that won't take the weight by making sure enough of them are flying at any given time. It doesn't work because the lift on the birds from their wings has an equal and opposite force on the floor of the van. For the van, it's a closed space so you know all the force will go to the floor of the van. For a plane, it isn't a closed space so the force can be much more spread out. It has to still exist, though - there are no exceptions to Newton's third law. --Tango (talk) 17:13, 19 August 2012 (UTC)
- Sure it exists, but its certainly important here to observe which objects this force is transferred to and what significant effects it can have with respect to other influences. In this case, with increases in the region affected, there is increasing mass involved thus there is less possible motion or speed. Moreover, one has to consider too that if I am getting out of bed or flying about, no net movement of the Earth or the atmosphere will occur if I have a twin doing the exact same thing, but creating an opposing force. In fact, such an equilibrium state and the observed lack of net influence is typical. Modocc (talk) 17:41, 19 August 2012 (UTC)
- So far as I know, transferring momentum always requires energy, but the amount of energy can get less and less. (Hence the "somewhat" I used above) For example, a photon can carry a quantum of angular momentum no matter how low frequency (low energy) it is, and as far as I know this doesn't set any minimum energy that photons can have. Likewise the momentum of a speeding bullet can be transferred to a stinging bruise behind a bulletproof vest, to the relatively mild force the person thus shot exerts on a wall to maintain his balance. Wnt (talk) 18:37, 19 August 2012 (UTC)
- When the plane's trailing downwash of air pressure has transferred its energy, the downwash seizes to exist. Its mass-energy and momentum is therefore conserved by the more massive mass-energy and momentum of the atmosphere and the Earth. --Modocc (talk) 19:17, 19 August 2012 (UTC)
- Yes, that "chickens in a sealed truck" thought experiment is an applicable one. Let's extend that by imagining a truck which is miles long, wide and high, with an airplane inside. Does the total system become lighter when the plane is in flight ? Obviously not. Therefore, there must be some downward force to provide the equivalent of the weight of the plane, at those times. I suggest that this is caused by higher pressure on the floor of the truck than on the roof. This, however, contradicts some of the statements above, such as the pressure dissipating evenly (above and below), or changing into heat. StuRat (talk) 20:44, 19 August 2012 (UTC)
- I believe what was meant above was that the plane's contribution to the pressure on the ground, which is force per area, is not going to be significant because it is spread over a wide area. -Modocc (talk) 21:39, 19 August 2012 (UTC)
- All the atmosphere's pressure is eventually transfered to the earth's surface, over the entire earth. Since the atmosphere has no lid, it is simply the atmosphere's total weight which presses on the earth. But air pressure is not the same as the coherent directional movement of air. Adding a plane of a certain weight to the atmosphere is just like adding an equivalent weight of air to the atmosphere. If a plane is actively flying, it is exerting a downward force on the air below its wings which may translate to the ground as wind, if it is close enough. But an airplane within a giant space will simply create circulating winds that will have no coherent direct action on the surface below it.
- Any increase in air pressure within a sealed container will exert just as much lifting force on the roof as it will depressing force on the floor. An airplane within an infinitely giant balloon would cause the balloon to expand, not to sink. Sustained flight within too small an enclosed area is simply not possible because the air will become turbulent and circulating currents will become harder and harder against which to generate lift. Within an infinitely large area you are dealing with just pressure--on the earth this is countered eventually by downward force--in an enclosed area it is countered by balanced increasing pressure on all surfaces in all directions. μηδείς (talk) 22:44, 19 August 2012 (UTC)
- For the most part I agree with you, because being trapped in a balloon, a bee or plane won't alter the balloon's buoyancy which is fixed, but the internal weight distribution can change with flight. Thus, when a plane takes off, it adds its weight to the atmosphere, the contribution of this force (its mass*g) being small... and if its within a giant balloon the balloon expands... which is from an increase in air pressure from the additional energy of the plane's propellants. When the plane lands, its mass-energy is correspondingly lighter and the atmosphere heavier. Its not all that difficult to hover within fairly confined spaces (think of a nearly buoyant swimmer treading water by pushing it downward) and wings produce pressures and partial pressures creating the winds of the downwash that, in turn, produce pressures, like sound waves and pressures against adjacent air masses as well as obstacles, but the essential impulse is usually rapidly absorbed by the surrounding large volume of air in a relatively short distance. Thus, the question of an atmospheric pressure change due to either the weight of the plane or due to the weight of the absorbed energy from a great altitude... seems to be similar to asking how much does the salinity of an ocean basin differs if one adds a grain of salt and ignores all the other contributions to its saltiness. That said, perhaps some tangible numbers, as the OP has requested, would clarify that this is indeed the case. -Modocc (talk) 03:58, 20 August 2012 (UTC)
- Suppose the plane flies around for a long time near the north pole. The gravity exerted by the plane will cause the Earth to be attracted to it with the same force as it attracts the plane, and over time, the Earth will fall constantly northward, accumulating a great deal of momentum (even if it is not particularly visible) in that direction --- unless, that is, some force presses back against the Earth with an equivalent force to that gravity. Now that press-back is not a universal inward force on the entire spherical surface of the Earth, because that wouldn't shove the Earth back in one direction away from the plane. It is a force that remains aligned with the position of the plane, transmitting its weight to the Earth somewhere vaguely below it, in such a way as to add up to precisely the right force to keep the Earth from being pulled by its own bootstraps in any particular direction simply because a plane is in the air. Wnt (talk) 04:16, 20 August 2012 (UTC)
- That's about right, for the Earth's momentum is conserved and does not and cannot change, and its precisely the force of the impulse that is involved, for it is absorbed, shoving the Earth and its atmosphere back, including regions above the plane, negating the gravitational pull. Without the Earth's gravity, the impulse would cause the Earth to be pushed southward. Again, an impulse that begins at a high altitude or even a modest altitude, is a comparatively very small force that spreads out over a very large area and becomes essentially absorbed and blended with other more significant interactions, for I think its likely to be a smaller influence than measurable localized barometric changes. We need to pin some numbers on this though. Modocc (talk) 07:01, 20 August 2012 (UTC)
- Suppose the plane flies around for a long time near the north pole. The gravity exerted by the plane will cause the Earth to be attracted to it with the same force as it attracts the plane, and over time, the Earth will fall constantly northward, accumulating a great deal of momentum (even if it is not particularly visible) in that direction --- unless, that is, some force presses back against the Earth with an equivalent force to that gravity. Now that press-back is not a universal inward force on the entire spherical surface of the Earth, because that wouldn't shove the Earth back in one direction away from the plane. It is a force that remains aligned with the position of the plane, transmitting its weight to the Earth somewhere vaguely below it, in such a way as to add up to precisely the right force to keep the Earth from being pulled by its own bootstraps in any particular direction simply because a plane is in the air. Wnt (talk) 04:16, 20 August 2012 (UTC)
- For the most part I agree with you, because being trapped in a balloon, a bee or plane won't alter the balloon's buoyancy which is fixed, but the internal weight distribution can change with flight. Thus, when a plane takes off, it adds its weight to the atmosphere, the contribution of this force (its mass*g) being small... and if its within a giant balloon the balloon expands... which is from an increase in air pressure from the additional energy of the plane's propellants. When the plane lands, its mass-energy is correspondingly lighter and the atmosphere heavier. Its not all that difficult to hover within fairly confined spaces (think of a nearly buoyant swimmer treading water by pushing it downward) and wings produce pressures and partial pressures creating the winds of the downwash that, in turn, produce pressures, like sound waves and pressures against adjacent air masses as well as obstacles, but the essential impulse is usually rapidly absorbed by the surrounding large volume of air in a relatively short distance. Thus, the question of an atmospheric pressure change due to either the weight of the plane or due to the weight of the absorbed energy from a great altitude... seems to be similar to asking how much does the salinity of an ocean basin differs if one adds a grain of salt and ignores all the other contributions to its saltiness. That said, perhaps some tangible numbers, as the OP has requested, would clarify that this is indeed the case. -Modocc (talk) 03:58, 20 August 2012 (UTC)
Ah, aerodynamics. The last remaining branch of classical physics where nobody can provide a coherent answer, but everyone thinks the answers are obvious except those who've actually studied the subject. But yes, ultimately a flying aircraft's weight is transferred to the ground via increased pressure. Take my word on it, I'm not an expert. ;)
As Modocc said, tangible numbers would help here. I'd start by looking into ground effect, where the pressure changes are going to be more obvious. AndyTheGrump (talk) 04:13, 20 August 2012 (UTC)
- Yes, "Ground effect", or just observing a low-flying plane or helicopter from just below, shows the force on the ground. Tango implies that pressure is always the same in all directions, and this is true in a static fluid, but is not true in a moving fluid such as the atmosphere. For a high-flying aircraft, the weight of the machine is ultimately exactly balanced by movement of air over a wide area, transferring an equal and opposite force to the surface of the earth on the side of the aircraft. By the time any pressure changes have been propagated to the far side of the earth, the aircraft will have landed (or completed several subsequent flights). As Andy implies, aerodynamics is very different from static pressure. When RAF planes fly low over my roof, I definitely feel the downward pressure! Dbfirs 11:58, 20 August 2012 (UTC)
Distance of galaxies from Earth
How many light-years from Earth would a congalaxy have to be if I made it a galaxy that hadn't been discovered by Terran astronomers yet? Subliminable (talk) 05:48, 19 August 2012 (UTC)
- What is a congalaxy? Someguy1221 (talk) 06:01, 19 August 2012 (UTC)
- This link should explain: Subliminable (talk) 06:10, 19 August 2012 (UTC)
- Working in proper lengths, the most distant observed galaxy-candidate is ~30 billion light years away (see Observable universe#Most distant objects). There's an extra ~17 billion light years of radius in the observable universe left to work with. Someguy1221 (talk) 06:24, 19 August 2012 (UTC)
- But what if I wanted to create a galaxy that was 600 million light years away? Would there be a realistic chance that science hadn't discovered such a galaxy yet as of 2012? Subliminable (talk) 06:29, 19 August 2012 (UTC)
- There are (estimated) over 100 billion galaxies in the observable universe, and the vast vast majority of them have never been identified. At 600 million light years you are probably safe. Someguy1221 (talk) 06:34, 19 August 2012 (UTC)
- Or you could place it so it couldn't be discovered. It would have to be hidden somehow. It could be behind a closer galaxy, or blocked by something within our own galaxy, like a gas nebula. StuRat (talk) 06:37, 19 August 2012 (UTC)
- On Stu's note, you could put it on the far side of the Milky Way's center, relative to Earth. Although that's not a position it could maintain, it would easily make it unobservable for the relatively brief period of time we've been using telescopes. Someguy1221 (talk) 06:41, 19 August 2012 (UTC)
- We are still discovering galaxies right at our doorstep, e.g. Ursa Major I Dwarf at 330000 light years. Youjust have to make them small enough. --Wrongfilter (talk) 07:32, 19 August 2012 (UTC)
- On Stu's note, you could put it on the far side of the Milky Way's center, relative to Earth. Although that's not a position it could maintain, it would easily make it unobservable for the relatively brief period of time we've been using telescopes. Someguy1221 (talk) 06:41, 19 August 2012 (UTC)
- Or you could place it so it couldn't be discovered. It would have to be hidden somehow. It could be behind a closer galaxy, or blocked by something within our own galaxy, like a gas nebula. StuRat (talk) 06:37, 19 August 2012 (UTC)
- We discover distant galaxies by picking a very small region of the sky and looking at it very closely. That means most galaxies haven't been discovered because they aren't in one of the regions we've looked at. --Tango (talk) 10:47, 19 August 2012 (UTC)
Electric Potential
Consider a circular disc of radius that lies in the xy plane and surface charge density (in cylindrical coordinates ): . Calculate the potential at a point .
This is my calculation:
The electric field is
.
Now, to calculate the potential: . Widener (talk) 10:51, 19 August 2012 (UTC)
- Do you have a question? -- Scray (talk) 12:07, 19 August 2012 (UTC)
- He wants us to check his method. Plasmic Physics (talk) 12:11, 19 August 2012 (UTC)
- Yeah. Widener (talk) 12:35, 19 August 2012 (UTC)
- My first question is, is the separation vector right, id est because is not even really a single particular vector in is it. That's a bit confusing. Widener (talk) 12:34, 19 August 2012 (UTC)
- He wants us to check his method. Plasmic Physics (talk) 12:11, 19 August 2012 (UTC)
- You're right, the separation vector is not correct. I don't understand either what is meant by since the direction of that unit vector depends on the coordinate of the point where the potential is being calculated and it seems to me that this coordinate was not given. Dauto (talk) 22:07, 19 August 2012 (UTC)
- Does it help if I mention that is small? Widener (talk) 23:58, 19 August 2012 (UTC)
- You're right, the separation vector is not correct. I don't understand either what is meant by since the direction of that unit vector depends on the coordinate of the point where the potential is being calculated and it seems to me that this coordinate was not given. Dauto (talk) 22:07, 19 August 2012 (UTC)
Is this stone is natural or human made?

This local people said that their ancestors worshiped this rock. They told this is a natural formation. But after saw the bottom side of the rock I think this is human made. Can any one guess this period? and artificial or natural? To see the bottom side of the rock exactly visit this blog.
http://fromthenmaduraitotenkasi.blogspot.in/2012/08/1.html
http://4.bp.blogspot.com/-ipo2kpQm7Ck/UC-wuyspMsI/AAAAAAAAAF8/JbT9pZqvkTM/s1600/IMG_0067.JPG
--Tenkasi Subramanian (talk) 17:42, 19 August 2012 (UTC)
- It's natural; see balancing rock. μηδείς (talk) 18:17, 19 August 2012 (UTC)
I already saw this balancing rocks. http://wowpics.in/amazing-pics/top-10-unbelivable-balanced-stone-around-the-world/
But after see the bottom side only I confused. Did u c the bottom side of the rock? see this link?
http://4.bp.blogspot.com/-ipo2kpQm7Ck/UC-wuyspMsI/AAAAAAAAAF8/JbT9pZqvkTM/s1600/IMG_0067.JPG
Can u say what type of bond is made between floor and rock?--Tenkasi Subramanian (talk) 18:23, 19 August 2012 (UTC)
- They're both just rock - by which I mean that the rock is continuous, its not actually balanced. Mikenorton (talk) 18:28, 19 August 2012 (UTC)
- You're saying it's a hoodoo? μηδείς (talk) 22:18, 19 August 2012 (UTC)
- No, there's no hard caprock here, just a layer that's slightly less resistant to erosion, which forms the narrow part of the rock. Still pretty strong rock though (compressive strength at least) to take that load. Mikenorton (talk) 07:16, 20 August 2012 (UTC)
- Looks natural to me but could have been enhanced by people? Really hard to tell without examining it for tool marks etc... PatHadley (talk) 09:50, 20 August 2012 (UTC)
- No, there's no hard caprock here, just a layer that's slightly less resistant to erosion, which forms the narrow part of the rock. Still pretty strong rock though (compressive strength at least) to take that load. Mikenorton (talk) 07:16, 20 August 2012 (UTC)
- You're saying it's a hoodoo? μηδείς (talk) 22:18, 19 August 2012 (UTC)
Ozone Dangers
Is it a fact that just 5% of Ozone if mixed with the atmosphere can cause all life-forms to vanish ? Also is it true that if ozone is cooled up to -200 , C liquidized, and put into a breakable glass ball, if blasted like a grenade can turn a man into glass? 124.253.89.68 (talk) 17:48, 19 August 2012 (UTC)
- Some one told that Ozone is O3 (Oxygen). I think that's not harmful.--Tenkasi Subramanian (talk) 18:29, 19 August 2012 (UTC)
- Please don't answer science desk questions based on what you 'think'. Check your facts:
- "Even very low concentrations of ozone can be harmful to the upper respiratory tract and the lungs. The severity of injury depends on both by the concentration of ozone and the duration of exposure. Severe and permanent lung injury or death could result from even a very short-term exposure to relatively low concentrations."
- I suggest you both read our Ozone article. As for the 'ozone grenade', where did you see that? AndyTheGrump (talk) 18:34, 19 August 2012 (UTC)
- Yes, as stated above, ozone is harmful if breathed in, but it's also quite helpful in blocking UV light in the ozone layer. So, we want lots of ozone in the air, just not down here where we would breath it. StuRat (talk) 20:24, 19 August 2012 (UTC)
- Just to clarify, we want lots as in ~5-10ppm, not ~5%. 203.27.72.5 (talk) 03:32, 20 August 2012 (UTC)
- Given it's half-life of 30 minutes in the lower atmosphere, I think fish in the sea would be safe and maybe some or most trees would survive. But it's highly corrosive and has a IDLH of 5ppm, making hydrogen cyanide with it's value of 50ppm seem quite benign in comparison. Found some rumours about India’s glass man, with a skeleton visible inside. Liquid ozone can detonate, but to turn a man into glass, you'd need a man made of silica to begin with ... Ssscienccce (talk) 23:23, 19 August 2012 (UTC)
- Glass has more than one meaning, and flash-frozen flesh meets the most general definition. μηδείς (talk) 23:29, 19 August 2012 (UTC)
- I did some back of the envelope calculations using the following data; density of liquid O3 = 1.352g/mL , heat capacity of liquid O3 ~ 0.45cal/gK, ozone boils at 161K, the latent heat of vapourization for O3 = 75.59 cal/g , and the heat capacity of O3 gas = 0.195 cal/gK ; and assumed that the grenade is a sphere of radius 5cm, it starts at 90K, the man weighs 100kg, his heat capacity is the same as water, his body temperature starts at 311K, to be considered "turned in glass" his whole body must be equal to or less than 273K, the grenade only cools the man and thermal equillibrium is reached instantly, and no exothermic oxidation reactions occur that would heat the system. 91542 calories are required to bring the grenade to 273K, and that's enought to cool 100000g by 0.91542K for a final temperature of about 37C. So, no this isn't possible just by flash freezing. If only his surface must be cooled, than you'd get a bit further with the flash freezing argument, but then you'd want to start correcting for the other generous assumptions made above. 203.27.72.5 (talk) 01:03, 20 August 2012 (UTC)
- Agreed, vitrification is possible, but not with a missile any man could heft in normal conditions. Better to use a hose. μηδείς (talk) 03:38, 20 August 2012 (UTC)
- I did some back of the envelope calculations using the following data; density of liquid O3 = 1.352g/mL , heat capacity of liquid O3 ~ 0.45cal/gK, ozone boils at 161K, the latent heat of vapourization for O3 = 75.59 cal/g , and the heat capacity of O3 gas = 0.195 cal/gK ; and assumed that the grenade is a sphere of radius 5cm, it starts at 90K, the man weighs 100kg, his heat capacity is the same as water, his body temperature starts at 311K, to be considered "turned in glass" his whole body must be equal to or less than 273K, the grenade only cools the man and thermal equillibrium is reached instantly, and no exothermic oxidation reactions occur that would heat the system. 91542 calories are required to bring the grenade to 273K, and that's enought to cool 100000g by 0.91542K for a final temperature of about 37C. So, no this isn't possible just by flash freezing. If only his surface must be cooled, than you'd get a bit further with the flash freezing argument, but then you'd want to start correcting for the other generous assumptions made above. 203.27.72.5 (talk) 01:03, 20 August 2012 (UTC)
smell vs taste
Why does tea smell wonderful when dry but taste bitter once made? (Please don't digress into overbrewing tea etc - I'm talking about the correctly made stuff.) Ta, 184.147.128.34 (talk) 17:51, 19 August 2012 (UTC)
- Coffee is the same way. And new tires smell good too, but I don't recommend eating them. ←Baseball Bugs carrots→ 18:49, 19 August 2012 (UTC)
- Tannin#Drinks with tannins seems to disagree with you as far as coffee having much for tannins. BigNate37(T) 19:57, 19 August 2012 (UTC)
- So, tannins are bitter, but don't have a particularly bitter smell. I've found that herbal teas (tisanes) often contain fewer tannins, if any, so aren't as bitter, since they don't contain any real tea. This is good, since it allows me to drink them without sweeteners, either real or artificial. StuRat (talk) 20:21, 19 August 2012 (UTC)
- Of course it's all completely subjective, I actually think the taste of straight tea is also wonderful, just like the smell. I don't like tea with sugar or milk added to it and I like it brewed quite strong. But I do occasionally enjoy a tea with lemon and honey, served hot on a cold day or cold on a hot day. Vespine (talk) 23:06, 19 August 2012 (UTC)
- To actually comment on the question, I think it's just because smell and taste receptors are actually quite different in some fundamental regards. Even though a lot of the time they seem to compliment each other, like a lot of fruit smells precisely how it expect it should taste, but in other cases, like salt for example doesn't really have much of a smell at all. You can basically tip a cup of salt into a pot of soup and it won't smell much different, but if you tasted it, it would be completely inedible. Vespine (talk) 23:14, 19 August 2012 (UTC)
- Of course it's all completely subjective, I actually think the taste of straight tea is also wonderful, just like the smell. I don't like tea with sugar or milk added to it and I like it brewed quite strong. But I do occasionally enjoy a tea with lemon and honey, served hot on a cold day or cold on a hot day. Vespine (talk) 23:06, 19 August 2012 (UTC)
- When I was kid, my brother baked brownies once. I first noticed when I smelled a beautiful intense aroma of vanilla wafting through the house. I came out into the kitchen to find out what he was doing and I asked him how much vanilla essence he put into the mix. He said that the recepie called for a cup but we didn't have that much so he just emptied the 50mL bottle that we had into the bowl. I had a look, and it actually called for a teaspoon. Needless to say, the brownies were probably the most bitter thing I've ever tasted. The look on his face when he bit into one of his creations was priceless. 203.27.72.5 (talk) 23:23, 19 August 2012 (UTC)
- You need to distinguish between taste, flavour, and aroma. When it comes to taste, we're talking about what your tongue detects, which isn't as basic as once thought (and doesn't involve that stupid tongue map), but is a lot more basic than smell. The main tastes are salty, sweet, bitter, sour, umami, with probably a few more that we don't really discuss much. These are tastes, not smells, so you detect them with your tongue: you might guess based on past experience that something that smells of strawberries will taste sweet, but if you're drinking a fruit tea your tastebuds will be disappointed. You cannot smell how something will taste, beyond extrapolating from past experience. Bitter is a taste, not a smell.
- When we actually eat something, we experience a flavour which is a combination of the taste and the smell/aroma. Because your nose and mouth are connected, you can smell the thing you are eating. When you drink a cup of tea, you taste the bitterness of the tannins which really shouldn't be much, if you're brewing the particular tea for the time it should be brewed for as well as experiencing the complicated combination of aromas from the tea that you could smell without drinking it: this combines to give a characteristic flavour.
- When we add artificial flavourings to food, these are aromatic compounds that make the food smell nice, affecting the flavour: altering the taste involves much more basic things, like altering the amount of salt, sugar, fat, caffeine, etc, which also alters the flavour. A strawberry has a different flavour to a peach because it smells like a strawberry: it has different aromatics. Both fruits taste sweet. 86.157.148.121 (talk) 13:07, 20 August 2012 (UTC)
- Just a silly story just in relation to how smell and taste are different, you mention fruit tea and I know exactly what you are talking about, but even more extreme example was this liquid fruit soap that I bought once which smelled exactly like delicious apples! But indeed tasted just like soap. Vespine (talk) 00:28, 21 August 2012 (UTC)
- Thank you 86.157.148.121 that was a great answer; I get it. 184.147.128.34 (talk) 09:16, 21 August 2012 (UTC)
August 20
Looking into the past
Is it possible in theory to look at the a part of the universe as it is now, and use that data to "predict" exactly how it was at a point in the past at a human scale? Could we learn exactly what the Earth was like down the last atom 100 million years ago, for example?--178.167.204.238 (talk) 01:07, 20 August 2012 (UTC)
- Absolutely, on a large scale. Just look at any object, like Pluto, and we can tell where it was in it's orbit back potentially billions of years. The same is true of stars and galaxies.
- Not at all, on the small scale. We don't know where each atom was 100 million years ago. StuRat (talk) 01:10, 20 August 2012 (UTC)
- Well, I understand we don't, but in theory could someone in the future with lots of resources and time gather enough data to work that out, or does something prevent that?--178.167.204.238 (talk) 01:21, 20 August 2012 (UTC)
- Entropy and black holes? AFAIK they technicaly don't destroy information, but i'm pretty sure they make it practically irretreivable. Vespine (talk)
- (ec x3)See Heisenberg uncertainty. It prevents the type of small scale back tracking you're talking about by preventing you from knowing the exact state of everything at any time, past, present or future. 203.27.72.5 (talk) 01:36, 20 August 2012 (UTC)
- (ec)The uncertainty principle makes it impossible to gather that much information. Whoop whoop pull up 01:38, 20 August 2012 (UTC)
- This idea is the basis for the slightly-reputable Omega Point theory by Frank J. Tipler. Staecker (talk) 02:33, 20 August 2012 (UTC)
Absolutely not! Forget about the necessary computational power, given no computer in the universe could possibly be complex enough to compute its own actions, plus those of the rest of the universe. See path independence, irreversible process, and Entropy (arrow of time) just to start.
- See also the works of Viscount Ilya Romanovich Prigogine. μηδείς (talk) 02:37, 20 August 2012 (UTC)
- I'm thinking theory can't prohibit everything, because (for example) it is possible that we scan the sky with a telescope and find an alien TV station broadcasting a video of their telescopic observations of the Earth 100 million years ago. Of course, that's not "the position of every atom", but it's a lot of detail. I doubt that there's any tiny particle, neutrino or otherwise, that can actually give this information if somehow you can observe a reflection or retransmission, but it doesn't seem conceptually impossible. See time viewer. Wnt (talk) 04:22, 20 August 2012 (UTC)
- Well, perhaps a better statement would be, current understanding is that no, it's not possible. 203.27.72.5 (talk) 04:33, 20 August 2012 (UTC)
- Well of course we could get aline TV, but even their TV wouldn't broadcast the action of each element on their homeworld. It is simply not possible to communicate X information without using X^N information, with N being some number above 1. μηδείς (talk) 04:36, 20 August 2012 (UTC)
- Well, perhaps a better statement would be, current understanding is that no, it's not possible. 203.27.72.5 (talk) 04:33, 20 August 2012 (UTC)
- I'm thinking theory can't prohibit everything, because (for example) it is possible that we scan the sky with a telescope and find an alien TV station broadcasting a video of their telescopic observations of the Earth 100 million years ago. Of course, that's not "the position of every atom", but it's a lot of detail. I doubt that there's any tiny particle, neutrino or otherwise, that can actually give this information if somehow you can observe a reflection or retransmission, but it doesn't seem conceptually impossible. See time viewer. Wnt (talk) 04:22, 20 August 2012 (UTC)
I remember a homework question in statistical mechanics that illustrated just how hard this is to do, even when considering purely classical systems. Chaotic systems, of which there are many classical examples, have the property that any uncertainty will grow exponentially with time. Suppose you wanted to know the evolution of every molecule of the atmosphere in a 1 m cube. This requires knowing not only the starting position and velocity of every particle but also all the external forces present to exquisite precision. There are 10 particles in that cubic meter, each moving at high speed and the future of each particle determined by the billiard-like collisions with other particles. Each collision magnifies the uncertainty as small differences in initial position lead to large changes in the new direction of travel after collision. One can calculate that even if you knew the initial positions and velocities exactly, and if you knew all the forces present (e.g. the precise details of gravity, electromagnetism, and everything else) to a precision of 10 N throughout the box, then that is only good enough to predict the path of the air molecules for a few minutes. After that, even this tiny error in specifying the forces present has been magnified to the point that you can no longer predict which molecules will collide and the whole thing becomes apparently random again. Because forces propagate over long distances, achieving even this few minutes of predictability would still require knowing every atom's position everywhere around the box for a distance of light years. And of course, this is considering only the purely classical parts of the problem, quantum mechanics will turn this practically impossible task into one that is truly impossible. Dragons flight (talk) 17:16, 21 August 2012 (UTC)
The physics and etc. of Total Recall (the 2012 remake)
I liked how the buildings looked in 2084, and was quite intrigued by "The Fall" super-elevator. I would hope their retirement homes by that time (as I'd likely live in one) would make a Chuck E. Cheese's look as boring as today's retirement homes by comparison.
Questions about The Fall:
F1) How could the difficulties of physics, the depth of the dig, et al., be overcome in order to build "The Fall" super-elevator that runs through the core of the planet?
F2) What "side-effects" would digging through the Earth's core cause?
F3) How much could "The Fall" cost, ballpark, to construct (in constant 2012 Euros?)
F4) How would the super-elevator be insulated from the core's excess heat & pressure?
F5) If any mechanical difficulty were to cause The Fall to stop somewhere in the shaft, many miles from either surface, what would be a suitable escape plan to bring the passengers back to the surface safely? (No point in ladders when you have 2,000+ miles to climb to a surface, unless they're also "tracks" for hyper-fast escape lifts.)
F6) To reach Australia from Britain in just 17 minutes, how could the acceleration and deceleration of The Fall be made gentle enough not to cause any significant ill effects for the passengers & crew?
F7) What would the terminal velocity be for an elevator big enough to hold 50,000 passengers and be as tall as a skyscraper?
F7.1) What would the propulsion methods have to be in order to race the elevator to the other side of the globe in just 17 minutes?
F8) Could the dig and installation be finished in just 72 years?
F9) What else could we plausibly invent in just 72 years to get from one end of the world to the other in 17 minutes?
F10) There was a scene where the characters opened an escape door and stepped outside during its ascent. Wouldn't there be some type of physical harm caused by the speed of the air flowing past you as fast as that elevator was going up?
Questions about the No Zones:
Z1) I was bothered that a global, chemical World War III could make much of the planet permanently uninhabitable. What kinds of chemicals would pollute the air in a non-dissipating fashion despite all sorts of wind, weather patterns, and the like? (They never dropped the "nuclear" term so I'd assume that no nuclear warfare took place.)
Z2) How effective would clean-up efforts be to clean the war's chemical pollution?
Z2.1) Why couldn't they keep cleaning up in order to make more living space available again?
Z2.2) If they had no problem building police "synthetics", why didn't I manage to see janitorial droids clean up the chemically-polluted wasteland?
Z2.3) How feasible could it be to get janitorial synthetics to clean up after our wars?
Z3) What new technologies could we invent to clean up after (that kind of) chemical warfare?
Z4) Above what height would the air have been safe above the "No-Zones?"
Z5) Why didn't I hear anything about undersea colonies? There is, after all, epic potential living space on and under the oceans.
Z5.1) If they have the engineering capability to construct "The Fall," how wouldn't they have the capability to build underwater, domed colonies? Amazingly enough, we already have something like this, albeit on a very small scale.
Miscellaneous "Total Recall" questions:
M1) Wouldn't the Holodeck be less invasive (no chemicals and tubes in arms, etc.) and overall, safer than the "Recall" chair?
M2) There was a scene where a tattoo was being worked on, that emitted varying types of light. What is the science and working behind luminescent tattoos?
M3) Would consumers really WANT a phone embedded in one's hand? Why not a glove-phone instead? Or a phone embedded on one's shirt/coat-sleeve? What other, future types of phones could we have for anyone who believes particular electronic implants could signify the Mark of the Beast?
M4) When living space is so scarce, why wouldn't that also spur us to build off-world colonies? We would have 72 years to make it feasible.
M5) How did The Fall become a "symbol of oppression?" And if it was, why wouldn't The Colony have prevented its construction in the first place?
M6) I noticed a Fiat Nuova 500 or two on the surface after the car descent scene. Given that it looks rather identical to the Nuova 500s made today, was there an antique car convention in town that day?
M7) Given that environmental movements (and their correlating tech advances) will make dead trees obsolete in 72 years, why would there still be a paper-based physical book-passport, as found in a bank deposit box?
B1) And as a bonus end-question, what other remakes are already in-theaters or are upcoming, that take place in a future? I was so fascinated by much of what I saw on this film. Thanks. --70.179.167.180 (talk) 04:51, 20 August 2012 (UTC)
- I renumbered your Q's to give each a unique number. StuRat (talk) 06:44, 20 August 2012 (UTC)
- F1) This part doesn't seem possible, at least in the time frame given. StuRat (talk) 07:46, 20 August 2012 (UTC)
- F9) Telepresence is probably the best way to get to the other side of the world instantly. You could hook yourself up to virtual reality equipment, and a robot on the other side of the world would send all the sounds, sights, smells, tastes, and tactile sensations to the VR equipment, where you would experience them, and also control the robot. Teleportation may also one day be possible, but perhaps not in that time frame. StuRat (talk) 06:46, 20 August 2012 (UTC)
- In the case of telepresence of 2 or more people, green screen technology could be used to make each person see the other when looking at their robot. StuRat (talk) 18:54, 20 August 2012 (UTC)
- Some parapsychologists believe that Teleportation is possible now, but they have been unable to demonstrate it to science. There is no logical reason to believe that this magic will become possible in future. Quantum teleportation specifically states that it does not involve reconstruction of an object (or person) at the other end, much to the disappointment of Star Trek fans. The Sci-Fi concept doesn't seem to have any basis in reality by current scientific and informatics understanding. We should have an article somewhere that explains why it is impossible. Dbfirs 12:12, 20 August 2012 (UTC)
- What is theoretically impossible about it ? It just involves scanning a person down to the molecular level, sending that information to the target location, synthesizing those molecules there, assembling them in the correct order, and "rebooting" the person (restarting their heart, etc.). Aside from the moral implications (no provision was made to transport the soul, so is destroying the original murder, with the copy being a clone ?), it all seems plausible to me. StuRat (talk) 19:00, 20 August 2012 (UTC)
- Some time ago, I read a good article explaining from an informatics viewpoint why it will never be possible. Unfortunately, I can't remember where I read it. The argument was on the lines that the amount of information required for reconstruction of a human is just too big for any machine that will fit in the universe, but I probably haven't expressed it very well. Can anyone find a good article? Dbfirs 19:41, 20 August 2012 (UTC)
- I suspect that they aren't allowing for proper data compression. For example, you don't need to send the position of every atom in every hemoglobin molecule. Just send the info that a hemoglobin molecule is located at positions X,Y,Z, and also send the detailed design of a typical hemoglobin molecule for that person (but only once). This could actually repair genetic damage, in that those hemoglobin molecules that are not typical would be replaced by those which are (so long as they remain close enough for the scanning device to figure out what they are supposed to be). StuRat (talk) 19:48, 20 August 2012 (UTC)
- Also, it isn't necessary to store all the info at once. You can scan one cell, send it's info on to the remote site, clear the buffer, scan the next cell, send it's info on, etc. At the remote site, they can get the info for one cell, create it, clear the buffer, get the info for the next cell, etc. StuRat (talk) 23:30, 20 August 2012 (UTC)
- So if you could make a copy of the person in this way, what happens to the original? 203.27.72.5 (talk) 20:30, 20 August 2012 (UTC)
- You either destroy it or you have an identical clone. This is the moral problem, either way. There was a (new) Outer Limits episode dealing with this moral issue: . StuRat (talk) 21:27, 20 August 2012 (UTC)
- I think the cell-by-cell method would take far too long for the duplicate to stay alive, but I can't find the article. I don't think I imagined it! Anyway, we agree that the concept has interesting moral implications, and, if possible at all, is not achievable by any method known to science at present. It will be interesting to see how long it is before 3D printers try to create a simple cell. Dbfirs 19:11, 21 August 2012 (UTC)
- Z1) Plenty of chemical pollutants last a long time, like CFCs, PCBs, and PBBs, and DDT. StuRat (talk)
- Z3) Nanites could search the world for pollutants, and convert them into something harmless, while also reproducing themselves. Genetically engineered bacteria might be another approach to do the same thing. StuRat (talk) 06:38, 20 August 2012 (UTC)
- Z5) I agree that undersea colonies would be a good idea. Unlike in space, both oxygen and water can be derived from sea water, temperature maintenance is easier, there's protection from the radiation and meteors of space, and potentially food can be derived from sea water as well (depending on how badly it was polluted). It also takes less energy and expense to move to and from the Earth's surface to there. Also, if they can build domes under the water, why not on the land, as well ? Presumably they could clean that small area of contaminants and keep it clean. StuRat (talk) 08:45, 20 August 2012 (UTC)
- M1) The Holodeck, as in STNG, requires teleportation technology to reposition you in the center of the room if you move near a wall. So, probably too ambitious for this time frame. Virtual reality could work, though. However, I think part of the selling point of the implanted memories is that you can feel as if you've spent a great deal of time on vacation, while actually not "wasting" all that time that could be spent in more productive activities. StuRat (talk) 08:00, 20 August 2012 (UTC)
- M2) There are bioluminescent substances, and they could be engineered to generate light from blood sugar. However, they wouldn't be very bright. You could probably only see them in the dark. StuRat (talk) 06:38, 20 August 2012 (UTC)
- M3) I'm with you here, although you could never forget your phone if it was part of you. Really, I think they are small enough now. What we need to work on is the functionality. Better cameras, better voice recognition, longer battery life, better reception, lower cost, etc. Incidentally, an electronic device can be too small. Watch calculators, for example, never really caught on. StuRat (talk) 07:33, 20 August 2012 (UTC)
- M6) Future cars are always problematic for movies. It's rather expensive to design an entirely new car for a movie, even with CGI. So, you often get a slightly modified current model. In this particular case, you might be looking at a case of product placement. StuRat (talk) 07:42, 20 August 2012 (UTC)
- M7) Electronics are nice, but you still want more permanent records of certain things. That wouldn't need to be paper, necessarily. I'm looking forward to a more permanent digital data format, some day. Perhaps a crystal with atoms replaced in certain lattice locations to represent bits of data. StuRat (talk) 07:40, 20 August 2012 (UTC)
- B1) (I didn't limit my answers to remakes.) You might want to see the movie Surrogates, if you haven't already. I assume you've seen The Matrix and it's sequels. I'd also recommend Bicentennial Man, A.I. Artificial Intelligence, and I, Robot. StuRat (talk) 06:33, 20 August 2012 (UTC)
- F6) Constant 1G acceleration is pretty bearable. It's what you've experienced in free fall any time you've ever fallen (or jumped). It wouldn't get you there in that time though. You'd need about 5G of acceleration and deceleration to make it there that fast. 203.27.72.5 (talk) 07:27, 20 August 2012 (UTC)
- Yes, and you could even go to 2g, which would feel like 1g, since 1g feels like no gravity at all, while in free fall. This would, however, require more energy. StuRat (talk) 07:44, 20 August 2012 (UTC)
- F7) If the elevator shaft was a vacuum there would be no terminal velocity. The elevator would accelerate until it reached the midpoint and gravity would slow it down from there. To complete the trip in 17 minutes, gravity is not sufficient however, and the midpoint velocity would be 89,964km/hr 203.27.72.5 (talk) 07:44, 20 August 2012 (UTC)
- F7.1) I don't know what the propulsion methods would be but a rail gun type system would see appropriate. As a side note, gravity alone would not get the elevator through the earth in 17 minutes. Even if you assume that you get 9.8m/s for the whole first half of the trip, after 8.5 minutes (510 seconds) you've only gone 1,274km of the 6371km of the Earth's radius. To get there in 17 minutes you'd have to accelerate at 49m/s (~5G), which causes disorientation, dizziness and fainting in humans. 203.27.72.5 (talk) 07:44, 20 August 2012 (UTC)
- F10) Ok, so if the elevator shaft was not a vacuum then yes there would be huge problems for anyone stepping out of the craft. If we assume that it was atmospheric pressure (so that he could breath in it) then you can compare it to this rail gun. The projectile there is moving at 9000km/hr. If the elevator has a uniform acceleration then it is travelling that fast 51 seconds after it leaves one side of the Earth. Note the unsurvivable ball of ionized gases in the linked picture. 203.27.72.5 (talk) 08:03, 20 August 2012 (UTC)
- However, the air in the shaft might also move with the capsule, particularly if it was the driving force in a pneumatic system. StuRat (talk) 08:11, 20 August 2012 (UTC)
- I haven't seen the movie but agree any plausible "Fall" would be in vacuum. Otherwise it's not that much better than flying there in a regular hypersonic airplane. But there's a vastly larger problem, which is that the Earth's core rotates relative to the surface. Though as long as you have magic hole-boring and -holding technology, maybe the impenetrable walls of your shaft stopped it. ... ...
- Implanted memories are indeed the true mark of the beast, and have vast social implications. You have a choice of two workers. Now Duh is a bright fellow, spent twelve years in grade school, four years undergrad, maybe five more getting a Ph.D., and now he's a novice expert in his field. Whereas Spiffy got the memory download of an expert when he was a little kid and has been working at it ever since. More importantly, Spiffy's artificial hippocampus downloaded the attitudes of the selected senior engineer, which include a once-rare sense of absolute loyalty and deference on personal issues that has since been tested in hundreds of thousands of workers conditioned with his thoughts and beliefs. Now if you're one of the six people who own the world, who are you going to hire, Duh or Spiffy? Wnt (talk) 13:54, 20 August 2012 (UTC)
- Also important is that if you did have this massively strong tube running through the centre of the Earth that could widthstand the movement of molten iron alloys in the core, it would disrupt the flow of those alloys to some degree, possibly enough to alter the power or orientation of the geodynamo that generates the Earth's magnetic field. 203.27.72.5 (talk) 21:10, 20 August 2012 (UTC)
- If the tube was not a vacuum, the air pressure would be higher than 1atm at great depths within it because the height of the column of air would be huge. If the pressures are higher, then there's more air there, so given the massive scale of the structure, building it might cause a significant fraction of the atmosphere to flow into it, lessening the amount we have up here. That would mean we have less protection from uncharged solar and cosmic radiation. Also, what happened to all of the rock that was pulled out of this hole? 203.27.72.5 (talk) 21:23, 20 August 2012 (UTC)
Relationship between electricity and intelligence
I have noticed that anything which could be described as an intelligent agent depends on electricity, including computers and the human brain. Is there any reason for this? Could you create a purely mechanical computer with an artificial intelligence? Widener (talk) 07:10, 20 August 2012 (UTC)
- I guess one reason is because electrons are nice and small so you can store lots more information with them than if you were to use larger particles. Widener (talk) 07:11, 20 August 2012 (UTC)
- Yes, and also quick. Mechanical adding machines were quite large and slow, by comparison. StuRat (talk) 07:19, 20 August 2012 (UTC)
- Indeed. A billiard-ball computer wouldn't be restricted to arithmetic-type operations. DMacks (talk) 07:24, 20 August 2012 (UTC)
- Here's a proper billard ball computer Giant Digicomp II. Dmcq (talk) 14:04, 20 August 2012 (UTC)
- I have one of the old Digicomp I machines. Wonder how many more I can find and multiplex and if it'll boot linux? DMacks (talk) 15:47, 20 August 2012 (UTC)
- Here's a proper billard ball computer Giant Digicomp II. Dmcq (talk) 14:04, 20 August 2012 (UTC)
- Indeed. A billiard-ball computer wouldn't be restricted to arithmetic-type operations. DMacks (talk) 07:24, 20 August 2012 (UTC)
- Although nowhere near being technologically achievable currently, it's at least theoretically possible to construct a purely mechanical computer which far outperforms current electronic computers in terms of gates per given volume, or in terms of instructions per second per watt. See the "Nanomechanical Computational Systems" chapter of the book "Nanosystems: Molecular Machinery, Manufacturing, and Computation" by K. Eric Drexler. Drexler's PhD thesis, which served as a rough draft of that book, can be read for free online. Red Act (talk) 15:35, 20 August 2012 (UTC)
- As far as whether it will ever be possible to actually construct such devices, see Drexler–Smalley debate on molecular nanotechnology. Red Act (talk) 19:34, 20 August 2012 (UTC)
- There are countless "things" you could notice that share a dependance on electricity, is there a reason for this? Probably because electricity is one of the fundamental forces. Also, I don't think anyone with a grasp of the topics involved would consider desribing computers as "intelligent agents".. But yes, there is nothing particularly special about "electricity" which gives it any unique intrensic properties. Essentially, all the electricity does is perform Boolean algebra, just really really fast. You may also be interested in Turing machine and Turing complete. Vespine (talk) 22:58, 20 August 2012 (UTC)
- As far as whether it will ever be possible to actually construct such devices, see Drexler–Smalley debate on molecular nanotechnology. Red Act (talk) 19:34, 20 August 2012 (UTC)
In megatons, what is the total estimated yield of the world's nuclear stockpile?
The title says it all. I'm certain that data exist on this somewhere (I'm fairly certain I've even heard numbers quoted, many years ago), but I've been unable to locate them. Evanh2008 08:19, 20 August 2012 (UTC)
- "The total global nuclear arsenal is about 30,000 nuclear warheads with a destructive capacity of 5,000 megatons or 5 gigatons (5,000 million tons) of TNT" from TNT_equivalent#Examples. No source is cited. 203.27.72.5 (talk) 08:31, 20 August 2012 (UTC)
- There are reasons to doubt that estimate — it is pretty out of date. The best estimates put the total stockpile size at 19,000 total, 4,800 operational. Awhile back I did a back-of-the-envelope guess based on published estimates from the Bulletin of the Atomic Scientists "Nuclear Notebook" series (which annually estimates the size and composition of various world nuclear stockpiles) and came up with a range of between 1.7 and 2.2 Gt, but if you made certain other assumptions you could get it up to 3.5 Gt plausibly. (I may have the numbers I used somewhere in an Excel spreadsheet, but not at hand.) The short answer is "nobody really knows, because the exact compositions of the world nuclear arsenals have not been disclosed, but it's probably a few gigatons." It's a lot less than it was during the Cold War, obviously, both because the number of weapons has been drastically cut back and the big nuclear states these days favor "just" large nukes (e.g. in the hundreds of kilotons range) rather than the ridiculously scary nukes of yore (megaton range). During the Cold War the "standard" estimates were around 5-10 gigatons. Note that just for comparison, all of the explosives expended during World War II, including the first atomic bombs, add up to just a few megatons. --Mr.98 (talk) 13:00, 20 August 2012 (UTC)
absorption of vitamins
Hello, I'd like to know if vitamins delivered through the digestion of vegetal cells were better absorbed than vitamins delivered “as is” through powder nutritional complements and fruit juice? Without protection, these vitamins will undergo the acidic digestion of the stomach and may be degraded. I know that some medical complements of vitamins (or of calcium) are administrated in non-digestible shells that open only when the intestine is reached, so as to protect its nutrients from the digestion of the stomach (the shells are then naturally excreted). 79.94.61.31 (talk) 09:20, 20 August 2012 (UTC)
- I thought the shells were digested somehow? I've been taking such vitamins for decades and have never seen shells in my excreta! --TammyMoet (talk) 17:55, 20 August 2012 (UTC)
- Unfortunately, the vitamin and supplement industry is very lucrative and very poorly regulated, a terrible combination. It's extremely common, almost universal that supplement manufacturers advertise using wild claims based on the flimsiest science, constantly flirting with the line between what is letigious and what just passes whatever little standard they are held up to. There is actually very little evidence that daily vitamin supplementation has any benefit what ever, unless you are actually malnourished or have a clinical deficiency. Basically, if you live in a 1st world country and get 3 meals a day, even if your diet isn't that great, you most likely won't actually get any benefit from dietary supplements. As to the question, yes, I'd be willing to bet that eating vegetables is almost certainly better for you in the long run then popping some pills. Vespine (talk) 23:12, 20 August 2012 (UTC)
- All I know is that I feel better when I take them than when I don't. Nowt wrong with a good placebo... --TammyMoet (talk) 08:14, 21 August 2012 (UTC)
- Well, there could be. It's possible to overdose on some vitamins and minerals, and they may contain sodium and sugar. Also, if they come from China, God knows what's in them. And, there are often multiple forms of a particular vitamin, some of which are easier to absorb than others. However, they often pick whichever form is cheapest. StuRat (talk) 08:25, 21 August 2012 (UTC)
- Checking a few vitamines, they all seem to be reasonably stable in acidic conditions. Some of them are acids themselves, and a few (like thiamine) come in the form of HCl salts (which are more stable than the free base form). Extended-release forms (like for high dose niacin) would by their nature protect (part of) their contents from exposure to stomach acid. Depending on essential nutrients that need protection from gastric acid might be an evolutionary dead end. Ssscienccce (talk) 23:47, 20 August 2012 (UTC)
Plutonium tetroxide
Is PuO4 known? If so, how is it synthesized (and what's the ref)? If not, how could it possibly be synthesized, or, if it's most likely not possible, why? Double sharp (talk) 10:14, 20 August 2012 (UTC)
- See here. Whoop whoop pull up 12:21, 20 August 2012 (UTC)
- I'm not a nuclear chemist, but the article seems to say: people have wondered if plutonium tetroxide could be synthesized, especially since NpO6 decays into PuO6; then follows some theoretical discussion, buffered by some experimental work, implying that it's possible that plutonium tetroxide could be made to work. "All these facts allow us to assume that the organic phase of nonpolar solvents contains neutral molecules of the previously undescribed compound of octavalent plutonium in the form of the corresponding tetroxide." My lay interpretation of the article is, "we think it can be done, we've no idea how to do it, even as experienced nuclear chemists, though maybe we've already found it but not proved it." --Mr.98 (talk) 13:09, 20 August 2012 (UTC)
Neuron Anatomy
I have been talking about Savant Syndrome with Dr Darold Treffert. The symptoms seem to be narrowed memory recall. Also he thinks Einstein is not autistic, he is a neuron typical genius. A person does not have to be autistic to be a savant and it could happen to anyone, including later acquired abilities. Which raised an interesting question, what is the anatomy of a savant brain? Is there any common structure that lead to the abilities? Would there be similarity to Kim Peek's brain? Did anyone do any research in this area or have any data to share? Thanks! -- RexRowan Talk 09:22, 20 August 2012 (UTC) Copied from Wikipedia_talk:WikiProject_Neuroscience#Neuron_Anatomy
canon 650d, Magic Lantern
I need to know which is the actual video recording bitrate of the 650d Someone know if there is magic lantern support for it in this moment? Thank you Iskánder Vigoa Pérez 15:52, 20 August 2012 (UTC) — Preceding unsigned comment added by Iskander HFC (talk • contribs)
- This has been asked and answered on the Computing desk. Rojomoke (talk) 16:22, 20 August 2012 (UTC)
Please add Vascepa (isosapent ethyl) to Misplaced Pages Encyclopedia
Hi, Vascepa (isosapent ethyl) was recently approved by the FDA as a triglyceride lowering medication. The company that manufactures it is Amarin. I believe it was approved late last month, July. Can you add this drug?
Thanks! — Preceding unsigned comment added by 68.11.125.232 (talk) 16:24, 20 August 2012 (UTC)
- We've had an "Ethyl eicosapentaenoic acid" article for many years now, and Icosapent is a redirect that points to it. DMacks (talk) 16:29, 20 August 2012 (UTC)
- I just now added a redirect for Vascepa. Red Act (talk) 17:05, 20 August 2012 (UTC)
maxwells inductance capacitance bridge
why we connect inductor in se & capacitor in parallel in maxwells inductance capacitance bridge — Preceding unsigned comment added by Sanathwiki (talk • contribs) 16:58, 20 August 2012 (UTC)
- This doesn't answer the question, but for reference the article on the topic is Maxwell bridge. Red Act (talk) 19:37, 20 August 2012 (UTC)
- It looks like R3 & L3 are actually the unknown (the component or components we want to measure) and the R3 and L3 in the schematic are representing the two properties of a physical inductor. A real world inductor has some finite internal resistance (the wire it is wound from has resistance) unlike the idealized model which could have inductance without resistance. That explains why R3 and L3 are in series. If we put the capacitor and resister in series would it work? Maybe (depending on frequency), but the formulas would be different and possibly not as nice to work with. (Sorry, In don't remmeber ever seeing the Maxwell bridge in school so I'm kind of guessing.) RJFJR (talk) 21:12, 20 August 2012 (UTC)
- If my understanding is correct, the Maxwell bridge has the cool property that the R2 and C2 settings that balance the bridge at one nonzero frequency will also balance the bridge at any other frequency, including zero. So it's possible to get the correct setting for R2 by using DC input and just adjusting R2, since the C2 setting won't affect things at DC. The R2 setting can then be left alone while just C2 is adjusted at some nonzero frequency. That same procedure wouldn't work if R2 and C2 were in series, and it'd be necessary to go back and forth between adjusting R2 and adjusting C2 to get the thing to balance, which wouldn't be nearly as convenient. The equations for R3 and L3 would also be more complicated in the altered circuit, since they would depend on the frequency. Red Act (talk) 22:06, 20 August 2012 (UTC)
- There are two important reasons for putting the resistor in parallel with the standard capacitor and not in series, and one minor reason. The first important reason is the one cited by Red Act - it makes the bridge balance independent of frequency - this means that a precision oscillator is not required. The second, just as important, reason, is that it makes for a much more convenient/practical resistance for R2. Consider a typical bridge with the standard capacitance of 1 μF (acurate values much above this are VERY expensive) and an energisation frequency of 1600 Hz - the reactance of the standard capacitance will be 100 ohms. Let's say we are measuring an inductance with a Q of 100 - a pretty ordianry value. To balance it, a series R2 would need to be 1.0 ohms. The slightest contact resistance (which after the bridge has seen some year's use might vary for 0.02 to 0.5 ohm from one setting to another) is a significant fraction of the total, and will make balancing a touchy and annoying task. But a parallel R2 will need a value of 10,000 ohms. This will swamp out any likely contact resistance over the service life of R2. The third, minor reason for a parallel R2 is that it also makes balance insensitive to any oscillator harmonics, so you can use a real cheap oscillator and an untuned detector. With a series R2, if you set it for balance at the fundamental, the bridge will be unbalanced for any oscillator harmonics, and the higher the harmonic the worse the unbalance. Keit58.164.239.117 (talk) 01:52, 21 August 2012 (UTC)
What's the percent of NASA projects that get canceled before completion?
Is it over 50%? I don't even raise an eyebrow when I hear about some project that is currently alive, because my perception is that whatever it is, the odds are in favor of the project being canceled before they actually do the thing the project sets out to do. That's my perception, at least. Does the data reflect this? 20.137.18.53 (talk) 17:47, 20 August 2012 (UTC)
- Almost by definition, zero programs are cancelled before completion. Whether certain specific goals of a specific project are attained is a different question. The way NASA and other government projects work is to outline very specific objectives, schedules, and funding programs. Once approved, the money is effectively "already spent." It's very difficult to "un-spend" that money. Projects or programs that are very successful are followed on by new projects with new funding. Less successful projects are rarely rewarded with follow-ons and new programs. High-level strategic objectives, like "putting a Mars sample-return program together," are not actually "projects" - they're strategic goals that evolve with time, and are supported by individual research and operations programs. So, the goal of a sample-return mission is never "cancelled;" but it can be re-evaluated from year to year, and it can be de-emphasized by changing the management of individual operational programs.
- You can see the full breakdown of all projects, and their budgets, here: NASA main budget webpage. Here's the "Performance Report", which quantitatively breaks down past and planned performance for programs and projects, including a lot of discussion about how to measure "success." Nimur (talk) 18:57, 20 August 2012 (UTC)
- If your definition of "cancel" is to not spend any money on a project, I don't think that's how most define it. In the case of NASA, stopping the project prior to (rocket) launch is what I would call cancellation. In the case of a project not involving a launch, like say funding a Neil deGrasse Tyson TV show, cancellation would mean that it is stopped before ready for broadcast, etc. StuRat (talk) 19:09, 20 August 2012 (UTC)
- Maybe I should have used the word 'mission' instead of project. Basically, the thing that gets pushed to the public as "Look what we're going to do!" 20.137.18.53 (talk) 19:08, 20 August 2012 (UTC)
- Take the example of Pluto Kuiper Express. That mission never launched. But, New Horizons did... so one can legitimately say that the mission to explore Pluto with a robotic probe was not cancelled. At the same time, certain line-items specific to the previous mission plan were cancelled. Two statements that seem, at least superficially, to contradict, are both true. So, unless you are willing to dig into the details, it's meaningless to talk about whether the "mission" was cancelled. NASA, like any large government organization, changes its plans, and is constrained by budget. Nimur (talk) 21:15, 20 August 2012 (UTC)
- OK, as for a slightly more specific subset of NASA "aspirations" (let that be a semantic umbrella term), how many times since the last time a human literally took off on a trip to the moon have they said "we're sending humans back by year X" only to have that specific plan be "modified" to "no, we're going to now send humans back by year Y," and so on, with perpetual constraint-induced inability to complete "aspirations" as currently specifically planned.? 67.163.109.173 (talk) 21:35, 20 August 2012 (UTC)
- I'm not trying to be needlingly pedantic, but you failed to explicitly state who "they" are. For example: in 2004, the President committed to a manned moon mission by 2020, but in the same report, you'll find that NASA administrator O'Keefe did not explicitly acknowledge that commitment. So, ... a question of political semantics: did NASA, in 2004, commit to a manned moon mission? If you believe yes, you might truthfully say that NASA has reneged (to be safe, you might wait until 2020 before). On the other hand it might be fair to say that NASA never committed to the former President's policy, so they can't be blamed for "cancelling" the objective. Nimur (talk) 22:01, 20 August 2012 (UTC)
- OK, if non-binding commitments released to the press by the President have no bearing to the agencies that are not at all committed by said "commitments," and are not worth the paper they're printed on, that makes it more difficult to actually believe anything a president or a politician or anyone not actually in a position of authority at the agency itself has to say about things supposedly to be accomplished by said agency. 67.163.109.173 (talk) 22:11, 20 August 2012 (UTC)
- My example was only to illustrate how subjective the answer to this question can be. In my opinion, a commitment is non-binding until there's money behind it. Even if the statement is made by the President. Nimur (talk) 22:14, 20 August 2012 (UTC)
- I guess all I'm saying then is if I were at NASA, I'd get pissed at politicians writing checks my butt can't cash for votes, knowing that a significant portion of vote-bearing citizens may not make the distinction that there's as much binding between the politician's words and NASA's acts as their (the voter's) own. Then when it never materializes long after the politician's gotten the short-term approval for saying the lofty things, people get pissed at NASA for not pulling a miracle out of their collective behinds by doing it without any money. 67.163.109.173 (talk) 23:12, 20 August 2012 (UTC)
- As a general rule, any commitment that a politician makes to do something which is to actually happen after their term in office has expired is just grandstanding. 203.27.72.5 (talk) 23:23, 20 August 2012 (UTC)
- The problem with that is that many NASA projects do require more than 8 years from conception to completion. So, if a politician starts a project and fully funds it during his term, I'd give him credit for that, even if the next administration cancels it. If, on the other hand, they announce grand plans, but have no intention of funding them during their term, then I agree with you. StuRat (talk) 02:50, 21 August 2012 (UTC)
- What about if the President comes up with a budget to fund it, along with a very large deficit and Congress rejects that budget and makes their own cutting down on stuff which includes this project, to reduce the deficit? Nil Einne (talk) 03:51, 21 August 2012 (UTC)
- Talk is cheap. The first Bush was going on about moon bases and Mars trips in 1989. I imagine that when his great-grandson is president, he'll still be promising Mars to the peasants to help whip a tax increase out of them, and invest it all in a penthouse in Rio de Janeiro. Wnt (talk) 12:12, 21 August 2012 (UTC)
- What about if the President comes up with a budget to fund it, along with a very large deficit and Congress rejects that budget and makes their own cutting down on stuff which includes this project, to reduce the deficit? Nil Einne (talk) 03:51, 21 August 2012 (UTC)
- The problem with that is that many NASA projects do require more than 8 years from conception to completion. So, if a politician starts a project and fully funds it during his term, I'd give him credit for that, even if the next administration cancels it. If, on the other hand, they announce grand plans, but have no intention of funding them during their term, then I agree with you. StuRat (talk) 02:50, 21 August 2012 (UTC)
- In case it isn't obvious to everybody reading this: the President of the United States can't actually give NASA any money to achieve a goal such as putting people on the Moon. The budget is entirely up to Congress. So, the President setting such a goal has no effect whatsoever, except insofar as it aids in convincing Congress to fund such a program.--Srleffler (talk) 16:28, 21 August 2012 (UTC)
- It cannot be used to compute a percentage but at Category:Cancelled space missions you can see what happened in some cases. PrimeHunter (talk) 13:15, 21 August 2012 (UTC)
- Thanks for the good laugh at Edward Makuka Nkoloso :) 20.137.18.53 (talk) 16:21, 21 August 2012 (UTC)
Unknown snake in the Pantanal
I took a photo of this snake crossing the Transpantaneira in the Pantanal. I tried to, but was unable to identify the species. Can anybody help? --Leyo 19:49, 20 August 2012 (UTC)
- Looks something like this blue mutation of the green tree snake, but with less yellow on the bottom at the front: . Not sure if those are native to Brazil, though. StuRat (talk) 20:25, 20 August 2012 (UTC)
- Green tree snakes are found in Australia and islands in this region. I forgot to mention that the snake was 2–3 meters long. --Leyo 20:36, 20 August 2012 (UTC)
- Could it be an invasive species in Brazil ? Or perhaps a related species ? Here's a better pic of a green tree snake (Dendrelaphis punctulatus): . StuRat (talk) 22:27, 20 August 2012 (UTC)
- It is not impossible that it is an invasive species, but I guess the probability is not too high. --Leyo 09:40, 21 August 2012 (UTC) PS. Most probably the species is on this list of Brazilian reptiles or this list for the Pantanal. There is also a book on the biota northern section of the Pantanal
- It has the rather prominent keel similar to that in sipos (genus Chironius), but I can't find any species with that coloration, except possibly the Amazonian whipsnake (Chironius exoletus). The crown ground snake (Liophis viridis) is closer in color but lacks the keel. Have you tried posting in herp forums? Someone might know enough about scale patterns and number to figure it out. ;) -- OBSIDIAN†SOUL 14:40, 21 August 2012 (UTC)
- There are two Chironius species, Chironius laurenti and Chironius quadricarinatus on this list. There are also five Liophis species on the list, but not the one you mentioned. --Leyo 17:16, 21 August 2012 (UTC)
- Congrats, you found it. :) It's Chironius laurenti. See bottom-most picture from the main page of Pouso Alegre.-- OBSIDIAN†SOUL 17:52, 21 August 2012 (UTC)
- There are two Chironius species, Chironius laurenti and Chironius quadricarinatus on this list. There are also five Liophis species on the list, but not the one you mentioned. --Leyo 17:16, 21 August 2012 (UTC)
- It has the rather prominent keel similar to that in sipos (genus Chironius), but I can't find any species with that coloration, except possibly the Amazonian whipsnake (Chironius exoletus). The crown ground snake (Liophis viridis) is closer in color but lacks the keel. Have you tried posting in herp forums? Someone might know enough about scale patterns and number to figure it out. ;) -- OBSIDIAN†SOUL 14:40, 21 August 2012 (UTC)
Cabbage and lettuce
How closely related are cabbage and lettuce? --168.7.239.105 (talk) 23:19, 20 August 2012 (UTC)
- Not very. Cabbage is a cruciferous vegetable, while lettuce is not. The most obvious difference to the consumer is that cabbage gives you gas, while lettuce does not. Cabbage is more closely related to Brussels sprouts. StuRat (talk) 23:24, 20 August 2012 (UTC)
- The relevant article is wild cabbage which was native in costal southern and western Europe: Brassica oleracea; cabbage, brussle sprouts, broccoli, cauliflower, kale, kholrabi and a few others are actulally just cultivars of the same plant, I've heard it described almost like dog breeds are clutivars of the same animal, not sure how loose that analogy is . Lettuce on the other hand was cultivated from weeds in Egypt. Vespine (talk) 00:22, 21 August 2012 (UTC)
- In taxonomic terms cabbage, both are generally considered core eudicots but cabbage is generally considered to be in the Rosids clade while lettuce in the Asterids clade . In other words their relation is fairly distant. While StuRat is correct cabbage is more closely related to brussel sprouts, as Vespine says, those are all very closely related, so closely related that usually they are considered the same species. To use other random examples to perhaps better illustrate the distance, lettuce is more closely related to the blueberries, cranberries, kiwifruit, azalea, potatoes, sweet potatoes, eggplant, petunia and forget-me-not while cabbage is more closely related to strawberries, apples, roses, cannabis, Rafflesia, pumpkin, watermelon, cucumber, pecan and walnut. Of course, being eudicots, they are more closely related to each other then they are to say rice, maize, onions, lilies, orchids and bananas. Nil Einne (talk) 01:13, 21 August 2012 (UTC)
August 21
Sticky Stuff Remover chemical identity
According to my online search, "Sticky Stuff Remover", for removing labels, has one active ingredient: CAS number 64742-47-8 - is this a mix of more than one chemical? Can it be obtained more economically in another form than "Sticky Stuff Remover"? --2.97.21.248 (talk) 02:39, 21 August 2012 (UTC)
- Most adhesives are oil soluble, so just about any oil will work. WD-40 works, but, if you want something more pleasant smelling, try peppermint oil. Be careful not to get it on plastic, though, as it may also dissolve that. StuRat (talk) 02:41, 21 August 2012 (UTC)
- CAS 64742-47-8 is a light petroleum distillate, so it's a mixture of low molecular weight hydrocarbons from crude oil. Apparently it's also sold as jet fuel , so I imagine that would be cheaper since you could buy it by the gallon. 203.27.72.5 (talk) 02:46, 21 August 2012 (UTC)
- Somehow I doubt if the corner gas/petrol/filling station has it. StuRat (talk) 02:47, 21 August 2012 (UTC)
- (edit conflict) Google turns up CAS number 64742-47-8 as "Hydrotreated light petroleum distillate" or other similar names. Here is one MSDS for it: . It is basically hydrogenated kerosene, that is middle-weight hydrocarbons (say 8-16 carbons) which have been treated with hydrogen to remove any unsaturation (double/triple bonds). --Jayron32 02:50, 21 August 2012 (UTC)
- Somehow I doubt if the corner gas/petrol/filling station has it. StuRat (talk) 02:47, 21 August 2012 (UTC)
What is a Gaussian belt?
I ran across the term Gaussian belt in the Radio source SHGb02+14a article. Cursory Google searches turned up a few examples of the phrase in astronomical contexts. Is it related to spectral width? Thanks. Braincricket (talk) 03:39, 21 August 2012 (UTC)
- It may be listed at List of things named after Carl Friedrich Gauss, but under a different name. Seriously, that dude has way too much stuff named after him. --Jayron32 04:58, 21 August 2012 (UTC)
- A full-text search on ADS yields a single scientific paper with the phrase "gaussian belt" and it's not relevant to this radio source. As far as I can see, the phrase in the article is meaningless. I've commented it out until someone provides a reference or an explanation what it's supposed to mean. --Wrongfilter (talk) 10:26, 21 August 2012 (UTC)
orchid
can anyone tellme the name of this orchid??? http://en.wikipedia.org/File:Small-orchids.jpg
thanks 04:29, 21 August 2012 (UTC)Iskánder Vigoa Pérez — Preceding unsigned comment added by Iskander HFC (talk • contribs)
- It's a Philippine ground orchid, Spathoglottis plicata. I have a picture of one in my backyard. :) -- OBSIDIAN†SOUL 04:49, 21 August 2012 (UTC)
- Too bad you only have a picture of one in your backyard. The actual flower would be even prettier. :-) StuRat (talk) 04:52, 21 August 2012 (UTC)
- Flowers wither, pictures are forever! ...and don't need watering. ;D -- OBSIDIAN†SOUL 06:35, 21 August 2012 (UTC)
- Too bad you only have a picture of one in your backyard. The actual flower would be even prettier. :-) StuRat (talk) 04:52, 21 August 2012 (UTC)
Estimate altitude from environmental conditions
I have pressure in torr, temperature in C and relative humidity. Is there a standard way of estimating an altitude from this information? Barometric formula, Atmospheric pressure and International Standard Atmosphere all have good information, but not quite what I'm looking for. I'm going to look around the office to see if we have a copy of the ISO spec for the ISA. Otherwise it's looking like I may have to derive it myself. 209.131.76.183 (talk) 12:41, 21 August 2012 (UTC)
- Formulas for estimating altitude are very fuzzy and tricky. The spherical cow approximations used in most calculations assume that the atmosphere is universal, constant, and unchanging: that is that there is a consistant set of atmospheric conditions whereby one could assume that altitude and air pressure are closely enough related to generate reliable data. That's how an altimeter works: it measures air pressure, assumes that surface air pressure is always the same value (it isn't) and that pressure decreases in a predictable way with altitude (again, if it does, it only does so in a very rough way). You're likely to be accurate to within a few hundred feet: for planes that's usually good enough to avoid hitting a mountain, as long as you've got enough of a buffer in your flight pattern, but we're still talking about an uncertainty of several percent, especially for smaller heights. Conceptually, what you're asking about is the correction between density altitude, pressure altitude, and actual altitude: technically feasible, as the density of air is related to the humidity and temperature (see Density of air for calculation) and air pressure is closely related to density. That density of air article has information on how humidity and temperature are used in calculating altitude. --Jayron32 12:56, 21 August 2012 (UTC)
- The International Standard Atmosphere and the American Standard Atmosphere both assume the atmosphere consists of dry air so they won't be of any help if you want to use relative humidity to help fix an altitude. Dolphin (t) 13:14, 21 August 2012 (UTC)
- I think I'm just going to throw out the temp and RH readings - looking more into how this is being used, I'm basically trying to simulate the output of a Kft altitude sensor that works entirely on pressure. Since the output I'm trying to duplicate doesn't care about temp and humidity, neither do I. I'll look for references on pressure to altitude, but if I don't find anything useful I'll take a stab at reversing the piecewise formula in Barometric formula. 209.131.76.183 (talk) 13:20, 21 August 2012 (UTC)
Mass of Thought
When I learn something new or remember an event, the information is stored in my brain. Does that information have mass?165.212.189.187 (talk) 13:38, 21 August 2012 (UTC)
- Your body as a whole will slightly lose mass when forming a thought or memory, as it takes work to do so. Not sure about your brain specifically, though. Goodbye Galaxy (talk) 13:58, 21 August 2012 (UTC)Irrelevant trolling?
- Reminds me of this Dilbert strip. AndrewWTaylor (talk) 15:04, 21 August 2012 (UTC)
- There is a physical component of memory (see Memory#Cognitive_neuroscience_of_memory), but you aren't adding new mass when you learn new things, I don't think. The physical basis of memory — how you get from an experience to an encoded set of neurons — is still a very nascent field of study, as I understand it. There's no doubt there's a physical basis, but it's unclear to me whether talking about that in terms of mass makes sense, except in the obvious sense that all things to do with your brain do have physical mass. The way I picture it, which is not especially scientific (and is a hash derived from various sources, particularly Antonio Damasio) is that memory is more like a rattling of electrical charges through an existing structure, and the more you do certain things, the stronger certain pathways get. So you might imagine the brain as a cluster of special wire where the more you use them, the easier they become to use. The wires themselves are not added or moved — it's solid state, more or less — and what you're doing is making some pathways become more used than others. "Used" here means the sending of electrical/chemical signals through them. Or something like that. Perhaps others will have better ways to explain such a thing. --Mr.98 (talk) 15:13, 21 August 2012 (UTC)
OK, well does information in general have mass? I am thinking more along the lines of the information paradox of black holes argument.165.212.189.187 (talk) 15:34, 21 August 2012 (UTC)
- As far as I know, from the age of three (?) we lose nerves every single day of our lives, so the brain should get lighter instead of heavier. Information is not stored in the brain as a physical entity, it is more like a connection. "The information is stored in my brain" is basically not correct. "The information can be recreated by my brain" would be a better way of phrasing this. Lova Falk talk 16:01, 21 August 2012 (UTC)
- A connection is a physical entity. (It is not a non-physical entity.) --Mr.98 (talk) 16:09, 21 August 2012 (UTC)
- As far as I know, from the age of three (?) we lose nerves every single day of our lives, so the brain should get lighter instead of heavier. Information is not stored in the brain as a physical entity, it is more like a connection. "The information is stored in my brain" is basically not correct. "The information can be recreated by my brain" would be a better way of phrasing this. Lova Falk talk 16:01, 21 August 2012 (UTC)
- Information is not a physical thing — it is better thought of as an event. It often is instantiated as a thing, but that is not its true nature. Consider a simple situation: I am signaling to you that I have arrived at my destination by flashing a laser at you. I've transmitted a bit of information. You might say, "well, you did shoot photons at me, and those are things." Indeed! (And note that we have there used massless things.) But if there had been an uninterrupted stream of photons, and I stopped them, now I'm signaling you with a lack of a thing. Thinking of information as a "thing" is just misleading — what matters is not whether a thing has been sent to you, or connected in your brain, or whatnot. What matters is the event. (Whether the event has meaning is a completely separate question!) Information theory is a nice place to start, but I warn you, it can get heavy sledding pretty quickly, because the technical definition of information (which matters if you are talking about black holes and things) is not the colloquial definition (i.e. semantic or conceptual information). --Mr.98 (talk) 16:07, 21 August 2012 (UTC)
Ok, I did check it out - double black diamonds! Thanks but wouldn't you have to preempt the "lack of thing to communicate" with a bunch of information communication to define what the lack of thing would actually communicate? So a connection is physical. Say the brain only uses 10% of its actual capacity, if it then was using 50% would that mean more connections...more mass than the 10% brain?165.212.189.187 (talk) 16:51, 21 August 2012 (UTC)
- Bekenstein bound#Human brain gives a limit, though not one presumed to be of any practical relevance. Wnt (talk) 17:35, 21 August 2012 (UTC)
What about this lizard
This photo was taken in Artemisa (Cuba) here these lizards are very ordinary, but I can’t find anyone that tell me it scientific name http://en.wikipedia.org/File:Brown_lizard.jpg — Preceding unsigned comment added by Iskander HFC (talk • contribs) 14:35, 21 August 2012 (UTC)
- The Cuban brown curly-tailed lizard, Leiocephalus cubensis -- OBSIDIAN†SOUL 15:01, 21 August 2012 (UTC)
(Chinese) Vegetable Identification
Can anyone identify this vegetable? It's common in Chinese grocery stores. Here's another photo.
Thanks! 2601:8:500:1B:8DFC:C408:2A71:54C0 (talk) 15:45, 21 August 2012 (UTC)pebble
- Looks like Bok Choy, known in the west as "cabbage". --Jayron32 15:52, 21 August 2012 (UTC)
(Doh never quite quick enough!!) It's usually called 'Pak Choi' in my local supermarket but seems to have a variety of names. The wikipedia Article Chinese cabbage is what comes up when you search pak choi in Misplaced Pages but the images don't seem to match with what I'd call Pak Choi..however if you do a google image search you'll find loads of pictures like yours. ny156uk (talk) 15:54, 21 August 2012 (UTC)
- We call it Pechay, and I grew up believing it was an English word.-- OBSIDIAN†SOUL 16:09, 21 August 2012 (UTC)
- See wikt:pak choi and wikt:白. Wnt (talk) 17:46, 21 August 2012 (UTC)
- That's funny. Many years ago I stopped in Angeles City on the way to Mount Pinatubo and saw curly/twister fries for the first time in my life. For years I believed they were indigenous. Perhaps they were. I never checked. Sean.hoyland - talk 18:08, 21 August 2012 (UTC)
- Hah. And I also thought Goldilocks Bakeshop was an American company (I mean jeez, the girl was blonde). :P -- OBSIDIAN†SOUL 18:16, 21 August 2012 (UTC)
- Marvelous. Sean.hoyland - talk 18:21, 21 August 2012 (UTC)
- Hah. And I also thought Goldilocks Bakeshop was an American company (I mean jeez, the girl was blonde). :P -- OBSIDIAN†SOUL 18:16, 21 August 2012 (UTC)
Radius of the universe
Ian Stewart's Concepts of Modern Mathematics contains the paragraph:
A certain theoretical physicist secured himself a mighty reputation on the basis of his deductions, on very general mathematical grounds, of a formula for the radius of the universe. It was a very impressive formula, liberally spattered with es, cs, hs and a few πs and √s for good measure. Being a theoretician, he never bothered to work it out numerically. It was several years before anybody had enough curiosity to substitute the numbers in it and work out the answer. Ten Centimeters.
Can someone tell me the name of the physicist and the formula? Thanks---Shahab (talk) 16:41, 21 August 2012 (UTC)
- It may very well be that this is merely a humorous anecdote (or, in common English, a "joke"), and is not intended to be a scrupulously correct historical assessment of an actual event. That is, it is meant to be illustrative of a common error in theoretical physics (that it exists in the abstract, without an attempt to provide actual experimentation or numbers), and not meant to be an actual historical example. In other words, it sounds like the kind of thing that a theoretical physicist would do (propose a formula and then not bother to check it). The actual size of the Universe (as a scientific fact) is discussed in the Misplaced Pages article and section Universe#Size.2C_age.2C_contents.2C_structure.2C_and_laws and is more fully fleshed out in articles linked from there. Historically, the physicist most commonly associated with our current understanding of the Universe and its actual size (or lack thereof) is Edwin Hubble, though I seriously doubt that he is the source of the above anecdote. Or indeed, that anyone may be. --Jayron32 16:52, 21 August 2012 (UTC)
- I've never heard this story before and it doesn't sound real. It sounds like a Hollywood hack writer's idea of theoretical physics. I tried googling and found only the book itself and a similar thread on physicsforums.com which reached no conclusion.
- There are cases of famous physicists making mistakes which went undetected for long periods of time, such as John von Neumann's hidden-variable proof of 1932 which was called into question 34 years later by J.S. Bell. I see the article mentions a 2010 paper claiming that von Neumann was right after all, but if he's right it means Bell's error wasn't discovered for 44 years, so this is still a good example. :-) -- BenRG (talk) 18:46, 21 August 2012 (UTC)
Multiple Big Bangs
Could more than one Big Bang have happened at the same time as ours? I realize that our concept of space doesn't apply to before then, but could a non-interacting bang have happened in the same "locaction", such that we can't detect it or that we can only see effects like dark matter or energy, but in themselves could house a perfectly viable universe, one where ours is the mysterious missing matter? Mingmingla (talk) 17:12, 21 August 2012 (UTC)
- No, I think this doesn't make sense. Dark matter and dark energy occupy the same spacetime as us, and one spacetime continuum means one big bang by any reasonable definition. There is a notion of particles that interact with each other but not at all (except gravitationally) with the particles we're made of, making them undetectable by anything other than dark-matter-like effects. This is sometimes called shadow matter, although that article seems to be about something slightly different. -- BenRG (talk) 18:54, 21 August 2012 (UTC)
Castleton Botanical Gardens
This garden or gardens are mentioned in several Misplaced Pages articles, including Jamaica and George Samuel Jenman, and Google gives several results, but the problem is that there are variations on the name, sometimes two in the same article. So, which of the following six variations is the actual name of the garden?
- Castleton Garden
- Castleton Gardens
- Castleton Botanic Garden
- Castleton Botanic Gardens
- Castleton Botanical Garden
- Castleton Botanical Gardens
Once I have the formal name, I can make it consistent within English Misplaced Pages.
- The nearest that I could find to an official webpage is The Jamaican Ministry of Agriculture and Fisheries Public Parks Department, which uses "Castleton Botanical Garden" in its header, although just to confuse matters it calls it "Castelton Gardens" in the following text. Alansplodge (talk) 19:36, 21 August 2012 (UTC)
Question about oral sex
Does the eating of semen affect your health in any way? Please answer as soon as possible, I've read that it causes cancer and I've been doing it for a while and it's ruined it all for me, I'm afraid of it now. — Preceding unsigned comment added by Alabamaboy1992 (talk • contribs) 20:06, 21 August 2012 (UTC)
Categories: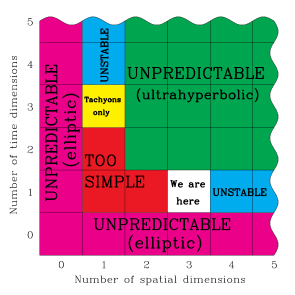
 is a constant (namely c) for straight-line motion, where x, y, z, t are coordinates defined by some inertial reference frame (a network of metersticks and Einstein-synchronized clocks).
is a constant (namely c) for straight-line motion, where x, y, z, t are coordinates defined by some inertial reference frame (a network of metersticks and Einstein-synchronized clocks).
 is their dissociation constant.
is their dissociation constant.
 . This seems pretty obvious (receptor concentration equals original/total concentration minus what is bound).
. This seems pretty obvious (receptor concentration equals original/total concentration minus what is bound). is really the ratio of bound to unbound hormone, and
is really the ratio of bound to unbound hormone, and  is the bound fraction. Now, if you compare your original equation to y = mx + b, can you see what you could learn from a Scatchard plot? --
is the bound fraction. Now, if you compare your original equation to y = mx + b, can you see what you could learn from a Scatchard plot? --  , ie:
, ie:  is unbound receptor, and
is unbound receptor, and  is the total receptor concentration, i.e.
is the total receptor concentration, i.e.  (but I am not the one who was looking at the book - I am making inferences). For an explanation of the
(but I am not the one who was looking at the book - I am making inferences). For an explanation of the  that lies in the xy plane and surface charge density (in cylindrical coordinates
that lies in the xy plane and surface charge density (in cylindrical coordinates  ):
):  . Calculate the potential at a point
. Calculate the potential at a point  .
.

 .
Now, to calculate the potential:
.
Now, to calculate the potential:  .
.  because
because  is it. That's a bit confusing.
is it. That's a bit confusing.  since the direction of that unit vector depends on the
since the direction of that unit vector depends on the  coordinate of the point where the potential is being calculated and it seems to me that this coordinate was not given.
coordinate of the point where the potential is being calculated and it seems to me that this coordinate was not given.  is small?
is small?