| Revision as of 14:20, 16 October 2014 editNikkimaria (talk | contribs)Autopatrolled, Extended confirmed users232,885 edits ref fixes← Previous edit | Revision as of 12:43, 17 October 2014 edit undo78.26 (talk | contribs)Autopatrolled, Administrators71,033 edits that's what the source says. (more precise)Next edit → | ||
| Line 1: | Line 1: | ||
| uril]], and uril]]<ref name=Dance />]]'''Molecular gyroscopes''' are ]s or ]es containing a ] that moves freely relative to a ], and therefore act as ]s. Though any ] or ] permits a ] to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a ] with low ]<ref name=Khuong /> or by physically surrounding the rotor avoiding ] contact.<ref name=Setaka /> A qualitative distinction can be made based on whether the ] needed to overcome rotational barriers is higher than the available ]. If the activation energy required is higher than the available thermal energy, the rotator undergoes "site exchange", jumping in steps between the distinct local energy minima on the ]. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotator can behave more like a ] freely rotating ] mass.<ref name=Khuong /> For example, several studies in 2002 with a ''p''-] rotor found that some structures using variable-temperature (VT) ] <sup>13</sup>C ] and ] ] were able to detect a two-site exchange rate of 1.6 MHz (over 10<sup>6</sup>/second at 65 °C), described as "remarkably fast for a phenylene group in a crystalline solid", with steric barriers of 12–14 ]/]. However, ] modification of the rotor increased the exchange rate to over 10<sup>8</sup> per second at room temperature, and the rate for inertially rotating ''p''-phenylene without barriers is estimated to be approximately 10<sup>12</sup> per second.<ref name=Khuong /> | uril]], and uril]]<ref name=Dance />]]'''Molecular gyroscopes''' are ]s or ]es containing a ] that moves freely relative to a ], and therefore act as ]s. Though any ] or ] permits a ] to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a ] with low ]<ref name=Khuong /> or by physically surrounding the rotor avoiding ] contact.<ref name=Setaka /> A qualitative distinction can be made based on whether the ] needed to overcome rotational barriers is higher than the available ]. If the activation energy required is higher than the available thermal energy, the rotator undergoes "site exchange", jumping in steps between the distinct local energy minima on the ]. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotator can behave more like a ] freely rotating ] mass.<ref name=Khuong /> For example, several studies in 2002 with a ''p''-] rotor found that some structures using variable-temperature (VT) ] <sup>13</sup>C ] and ] ] were able to detect a two-site exchange rate of 1.6 MHz (over 10<sup>6</sup>/second at 65 °C), described as "remarkably fast for a phenylene group in a crystalline solid", with steric barriers of 12–14 ]/]. However, ] modification of the rotor increased the exchange rate to over 10<sup>8</sup> per second at room temperature, and the rate for inertially rotating ''p''-phenylene without barriers is estimated to be approximately 2.4 x 10<sup>12</sup> per second.<ref name=Khuong /> | ||
| {{clear}} | {{clear}} | ||
Revision as of 12:43, 17 October 2014
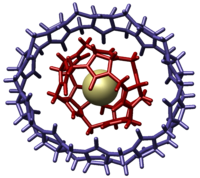
Molecular gyroscopes are chemical compounds or supramolecular complexes containing a rotator that moves freely relative to a stator, and therefore act as gyroscopes. Though any single bond or triple bond permits a chemical group to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a crystal structure with low packing density or by physically surrounding the rotor avoiding steric contact. A qualitative distinction can be made based on whether the activation energy needed to overcome rotational barriers is higher than the available thermal energy. If the activation energy required is higher than the available thermal energy, the rotator undergoes "site exchange", jumping in steps between the distinct local energy minima on the potential energy surface. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotator can behave more like a macroscopic freely rotating inertial mass. For example, several studies in 2002 with a p-phenylene rotor found that some structures using variable-temperature (VT) solid-state C CPMAS and quadrupolar echo H NMR were able to detect a two-site exchange rate of 1.6 MHz (over 10/second at 65 °C), described as "remarkably fast for a phenylene group in a crystalline solid", with steric barriers of 12–14 kcal/mol. However, tert-butyl modification of the rotor increased the exchange rate to over 10 per second at room temperature, and the rate for inertially rotating p-phenylene without barriers is estimated to be approximately 2.4 x 10 per second.
| Year of publication | Rotator | Stator | Linkage | Reference |
|---|---|---|---|---|
| 2002 | cucurbituril | cucurbituril | noncovalent | |
| 2007 | p-phenylene | two m-methoxy-substituted trityl groups | triple bonds | |
| 2007 | p-phenylene | triply bridged trityl cage | triple bonds | |
| 2010 | halogen-substituted p-phenylene | silaalkane chains | single bonds | |
| 2014 | p-phenylene | trityl groups bridged by photoactive azobenzene bridge | triple bonds |
References
- ^ Day, Anthony I.; Blanch, Rodney J.; Arnold, Alan P.; Lorenzo, Susan; Lewis, Gareth R.; Dance, Ian (2002). "A Cucurbituril-Based Gyroscane: A New Supramolecular Form". Angew. Chem. Int. Ed. 41 (2): 275–277. doi:10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Tinh-Alfredo V. Khuong, Hung Dang, Peter D. Jarowski, Emily F. Maverick, and Miguel A. Garcia-Garibay (2007). "Rotational Dynamics in a Crystalline Molecular Gyroscope by Variable-Temperature C NMR, H NMR, X-Ray Diffraction, and Force Field Calculations" (PDF). J. Am. Chem. Soc. 129 (4): 839–845. doi:10.1021/ja064325c.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wataru Setaka, Soichiro Ohmizu, Mitsuo Kira (2010). "Molecular Gyroscope Having a Halogen-substituted p-Phenylene Rotator and Silaalkane Chain Stators". Chemistry Letters. 39 (5): 468–469. doi:10.1246/cl.2010.468.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Jose E. Nuez, Arunkumar Natarajan, Saeed I. Khan, and Miguel A. Garcia-Garibay (2007). "Synthesis of a Triply-Bridged Molecular Gyroscope by a Directed Meridional Cyclization Strategy" (PDF). Org. Lett. 9 (18): 3559–3561. doi:10.1021/ol071379y.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Patrick Commins and Miguel A. Garcia-Garibay (2014). "Photochromic Molecular Gyroscope with Solid State Rotational States Determined by an Azobenzene Bridge". J. Org. Chem. 79 (4): 1611–1619. doi:10.1021/jo402516n.
This chemistry-related article is a stub. You can help Misplaced Pages by expanding it. |