| Revision as of 19:59, 6 December 2013 editDraphoen (talk | contribs)2 editsNo edit summary← Previous edit | Revision as of 00:58, 24 October 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,701 edits add links, replace image with R with real derivative, add doi and another book ref to the area, shorten some parts of lede to keep focus on topicNext edit → | ||
| Line 1:
], is the parent member.]]
| |||
Revision as of 00:58, 24 October 2014

A rylene dye is a dye based on the perylene framework. In homologues additional naphthalene units are added, forming compounds - or poly(peri-naphthalene)s - such as terrylene, quarterrylene.
Perylene dyes are useful for their intense visible light absorption, high stability, electron accepting ability, and unity quantum yields. Due to these properties, they are actively researched in academia for optoelectronic and photovoltaic devices, thermographic processes, energy-transfer cascades, light-emitting diodes, and near-infrared-absorbing systems. A bathochromic shift of about 100 nm is recorded per additional naphthalene unit.
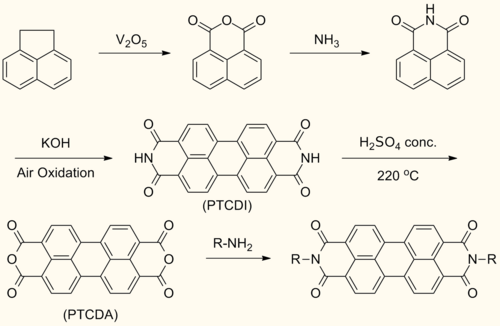
Synthesis
Perylenediimide (PDI) are synthesized by treating perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) with amines at high temperatures.
This reaction forms symmetrically N,N’- substituted PDIs. Solubilizing groups are often attached in this fashion. Although the solubility of the dianhydride is low, the solubility of some mono- and diimide derivatives are greatly improved.
Applications
Pigments
Perylene diimide derivatives were initially developed as industrial dyes due to their excellent chemical, photo, thermal, and weather stability. Nowadays, perylene dyes are used predominantly in textile applications and as high-grade industrial paint.

Several perylene pigments have been developed for artist's paints :
- Pigment red 149, a middle red, between light and dark cadmium red. (chemical structure: replace HN group in Pigment violet 29 with 3,5-CH3N)2C6H3).
- Pigment Red 179, which is close to alizarin crimson (structure: replace HN group in Pigment violet 29 with CH3N).
close to alizarin crimson (PR83).
- Pigment Red 29 (chemical structure: see image above)
- Perylene black 31 (replace HN group in Pigment violet 29 with C6H5CH2CH2N)
Protein Tagging
Rylene dyes have been less popular as a fluorescent tag due to its low solubility in aqueous solutions. However, there has been much progress in tailoring rylene probes to this application. An advantage of using rylene dyes is that different functional groups can be placed in the imide structure and in the bay region. This has allowed polar carboxylic acid and sulfonic acid groups to be added to make rylene dyes more soluble. The imide structure allows for two different substituent, so a single reactive group can be attached to this position.
Organic Field Effect Transistors
Perylene diimide derivatives can be used as n-channel field-effect transistors due to their strong electron affinities. In particular, OFETs using highly packed perylene diimide derivatives with electron withdrawing groups such were found to have high air stability. Close molecular packing was found to be an important factor in retaining air stability. Electron transfer, on the other hand, was strongly influenced by the π-π stacking (link to Stacking (chemistry)) between perylene diimide units.
Organic Solar Cells
Perylene diimide derivatives are a good candidate as acceptor materials due to their high electron affinity and high electron mobilities. Highly stable organic solar cells with high electron mobility have been reported in scientific literature. The HOMO-LUMO levels (link to HOMO/LUMO) of perylene diimide derivatives can easily be tuned via substitution at the bay position.
References
- ^ Huang, C., Barlow,S., Marder, S. (2011), Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. Journal of Organic Chemistry, 76, 2386–2407
- ^ Weil, T., Vosch, T., Hofkens, J., Peneva, K. and Müllen, K. (2010), The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angewandte Chemie International Edition, 49: 9068–9093. doi:10.1002/anie.200902532
- K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- Greene, M. "Perylene Pigments" in High Performance Pigments, 2009, Wiley-VCH, Weinheim. doi:10.1002/9783527626915.ch16 pp. 261-274.
- Jones, B. A.; Ahrens, M. J.; Yoon, M.-H.; Facchetti, A.; Marks, T. J.; Wasielewski, M. R. (2004) Electron transport: High-mobility air-stable n-type semiconductors with processing versatility: dicyanoperylene-3,4:9,10-bis(dicarboximides). Angew. Chem., Int. Ed., 43, 6363– 6366
- Mei, J., Diao, Y., Appleton, A. L., Fang, L., Bao, Z. (2013), Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 135, 6724
- Usta, H., Facchetti, A., Marks, T. J. (2011) n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 44, 501−510