| Revision as of 17:39, 2 April 2021 editLegionMammal978 (talk | contribs)Extended confirmed users7,894 editsm capitalized name← Previous edit |
Latest revision as of 19:22, 30 January 2024 edit undoOlliverWithDoubleL (talk | contribs)Extended confirmed users24,715 edits short description, links, nowrap on infobox titleTags: Mobile edit Mobile app edit iOS app edit |
| (One intermediate revision by one other user not shown) |
| Line 1: |
Line 1: |
|
{{short description|Chemical compound}} |
|
{{short description|Organic molecule (C18H12) made of four fused benzene rings}} |
|
{{DISPLAYTITLE:Benzo(''c'')phenanthrene}} |
|
{{DISPLAYTITLE:Benzo(''c'')phenanthrene}} |
|
{{correct title|reason=bracket|edit=substitution|Benzophenanthrene}} |
|
{{correct title|reason=bracket|edit=substitution|Benzophenanthrene}} |
| Line 6: |
Line 6: |
|
| Watchedfields = changed |
|
| Watchedfields = changed |
|
| verifiedrevid = 437570118 |
|
| verifiedrevid = 437570118 |
|
| Name = Benzophenanthrene |
|
| Name = {{nowrap|Benzophenanthrene}} |
|
| ImageFileL1 = Benzo(c)phenanthrene.png |
|
| ImageFileL1 = Benzo(c)phenanthrene.png |
|
| ImageFileR1 = Benzo(c)phenanthrene-3D-balls.png |
|
| ImageFileR1 = Benzo(c)phenanthrene-3D-balls.png |
| Line 51: |
Line 51: |
|
}} |
|
}} |
|
|
|
|
|
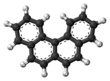
'''Benzophenanthrene''' is a ] with the ] C<sub>18</sub>H<sub>12</sub>. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule<ref>{{Cite journal|last=Hirshfeld|first=F. L.|last2=Sandler|first2=Selina|last3=Schmidt|first3=G. M. J.|date=1963-01-01|title=398. The structure of overcrowded aromatic compounds. Part VI. The crystal structure of benzophenanthrene and of 1,12-dimethylbenzophenanthrene|journal=Journal of the Chemical Society (Resumed)|language=en|pages=2108|doi=10.1039/jr9630002108|issn=0368-1769}}</ref><ref>{{cite journal |first1=M. |last1=Levy |first2= Melvin S. |last2= Newman |first3= M. |last3= Szwarc |title= Methyl Affinities of Non-planar Aromatic Hydrocarbons |journal= J. Am. Chem. Soc. |year= 1955 |volume= 77 |issue= 16 |pages= 4225–4225 |doi= 10.1021/ja01621a015}}</ref> consisting of the fusion of four fused ] rings. The compound is of mainly theoretical interest but it is environmentally occurring<ref>{{Cite journal|last=Tolosa|first=Imma|last2=de Mora|first2=Stephen|last3=Sheikholeslami|first3=Mohammad Reza|last4=Villeneuve|first4=Jean-Pierre|last5=Bartocci|first5=Jean|last6=Cattini|first6=Chantal|date=2004-01-01|title=Aliphatic and aromatic hydrocarbons in coastal caspian Sea sediments|journal=Marine Pollution Bulletin|volume=48|issue=1–2|pages=44–60|doi=10.1016/S0025-326X(03)00255-8|pmid=14725875}}</ref> and weakly ]ic.<ref>{{Cite journal|last=Hu|first=X.|last2=Xia|first2=H.|last3=Srivastava|first3=S. K.|last4=Pal|first4=A.|last5=Awasthi|first5=Y. C.|last6=Zimniak|first6=P.|last7=Singh|first7=S. V.|date=1998-12-01|title=Catalytic efficiencies of allelic variants of human glutathione S-transferase P1-1 toward carcinogenic anti-diol epoxides of benzophenanthrene and benzochrysene|journal=Cancer Research|volume=58|issue=23|pages=5340–5343|issn=0008-5472|pmid=9850062}}</ref> |
|
'''Benzophenanthrene''' is a ] with the ] {{chem2|C18H12}}. It is a white solid that is soluble in nonpolar ]s. It is a ]<ref>{{Cite journal|last1=Hirshfeld|first1=F. L.|last2=Sandler|first2=Selina|last3=Schmidt|first3=G. M. J.|date=1963-01-01|title=398. The structure of overcrowded aromatic compounds. Part VI. The crystal structure of benzophenanthrene and of 1,12-dimethylbenzophenanthrene|journal=Journal of the Chemical Society (Resumed)|language=en|pages=2108|doi=10.1039/jr9630002108|issn=0368-1769}}</ref><ref>{{cite journal |first1=M. |last1=Levy |first2= Melvin S. |last2= Newman |first3= M. |last3= Szwarc |title= Methyl Affinities of Non-planar Aromatic Hydrocarbons |journal= J. Am. Chem. Soc. |year= 1955 |volume= 77 |issue= 16 |pages= 4225 |doi= 10.1021/ja01621a015}}</ref> consisting of the ] of four fused ] rings. The compound is of mainly theoretical interest but it is environmentally occurring<ref>{{Cite journal|last1=Tolosa|first1=Imma|last2=de Mora|first2=Stephen|last3=Sheikholeslami|first3=Mohammad Reza|last4=Villeneuve|first4=Jean-Pierre|last5=Bartocci|first5=Jean|last6=Cattini|first6=Chantal|date=2004-01-01|title=Aliphatic and aromatic hydrocarbons in coastal caspian Sea sediments|journal=Marine Pollution Bulletin|volume=48|issue=1–2|pages=44–60|doi=10.1016/S0025-326X(03)00255-8|pmid=14725875}}</ref> and weakly ]ic.<ref>{{Cite journal|last1=Hu|first1=X.|last2=Xia|first2=H.|last3=Srivastava|first3=S. K.|last4=Pal|first4=A.|last5=Awasthi|first5=Y. C.|last6=Zimniak|first6=P.|last7=Singh|first7=S. V.|date=1998-12-01|title=Catalytic efficiencies of allelic variants of human glutathione S-transferase P1-1 toward carcinogenic anti-diol epoxides of benzophenanthrene and benzochrysene|journal=Cancer Research|volume=58|issue=23|pages=5340–5343|issn=0008-5472|pmid=9850062}}</ref> |
|
|
|
|
|
==References== |
|
==References== |