| Revision as of 18:16, 20 October 2023 editMichael7604 (talk | contribs)Extended confirmed users8,895 editsmNo edit summary← Previous edit | Latest revision as of 22:08, 27 September 2024 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,697 edits 10000 cites in CA, so I added two | ||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 21: | Line 21: | ||
| | Appearance = White to pale yellow crystals<ref name=goodscents>, The Good Scents Company</ref> | | Appearance = White to pale yellow crystals<ref name=goodscents>, The Good Scents Company</ref> | ||
| | Density = 1.094 g/cm<sup>3</sup> | | Density = 1.094 g/cm<sup>3</sup> | ||
| | MeltingPtC = 38. |
| MeltingPtC = 38.2 | ||
| | MeltingPt_ref = <ref name= |
| MeltingPt_ref = <ref name=Pubchem>{{PubChem|7476|4'-Methoxyacetophenone}}</ref> | ||
| | BoilingPtC = |
| BoilingPtC = 254 | ||
| | BoilingPt_ref = <ref name= |
| BoilingPt_ref = <ref name=Pubchem/> | ||
| | Solubility = 2470 mg/L<ref name= |
| Solubility = 2470 mg/L<ref name=goodscents/> | ||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| Line 34: | Line 34: | ||
| }} | }} | ||
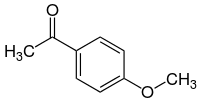
| '''Acetanisole''' is an ] ] with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition acetanisole can sometimes smell like butter or caramel. |
'''Acetanisole''' is an ] ] with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition acetanisole can sometimes smell like butter or caramel.<ref name=Aldrich/> Its chemical names are based on considering the structure as either an ] (]-]) analog of ]. Other names It can also be seen as a ] analog of ]. | ||
| Acetanisole is found naturally in ], the glandular secretion of the ].<ref name="goodscents"/> | Acetanisole is found naturally in ], the glandular secretion of the ].<ref name="goodscents"/> | ||
| ==Preparation== | ==Preparation== | ||
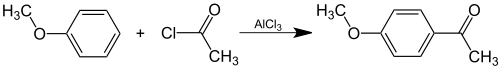
| Acetanisole can be prepared ] by ] of |
Acetanisole can be prepared ] by ] of anisole with ]: | ||
| :] | :] | ||
| ==Application== | ==Application== | ||
| It is used as a ] |
It is used as a ],<ref> {{webarchive|url=https://web.archive.org/web/20080411182413/http://tobaccodocuments.org/profiles/additives/acetanisole.html|date=April 11, 2008}}</ref> a fragrance,<ref name=goodscents/> and a flavoring in ].<ref>{{CodeFedReg|21|172|515}}</ref> | ||
| ==Reactions== | |||
| 4-Methoxyacetophenone is a standard substrate or product of much research, such as ]<ref>{{cite journal |doi=10.1021/jo010721w |title=Metal−Ligand Bifunctional Catalysis: A Nonclassical Mechanism for Asymmetric Hydrogen Transfer between Alcohols and Carbonyl Compounds |date=2001 |last1=Noyori |first1=Ryoji |last2=Yamakawa |first2=Masashi |last3=Hashiguchi |first3=Shohei |journal=The Journal of Organic Chemistry |volume=66 |issue=24 |pages=7931–7944 |pmid=11722188 }}</ref> and directed arylations.<ref>{{cite journal |doi=10.1021/ja972593s |title=Palladium-Catalyzed α-Arylation of Ketones |date=1997 |last1=Palucki |first1=Michael |last2=Buchwald |first2=Stephen L. |journal=Journal of the American Chemical Society |volume=119 |issue=45 |pages=11108–11109 }}</ref> | |||
| == References == | == References == | ||
Latest revision as of 22:08, 27 September 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name 1-(4-Methoxyphenyl)ethan-1-one | |
| Other names 4-Acetylanisole; para-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.002.560 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H10O2 |
| Molar mass | 150.177 g·mol |
| Appearance | White to pale yellow crystals |
| Density | 1.094 g/cm |
| Melting point | 38.2 °C (100.8 °F; 311.3 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Solubility in water | 2470 mg/L |
| Hazards | |
| Flash point | 138 °C (280 °F) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Acetanisole is an aromatic chemical compound with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition acetanisole can sometimes smell like butter or caramel. Its chemical names are based on considering the structure as either an acetyl (methyl-ketone) analog of anisole. Other names It can also be seen as a methyl ether analog of acetophenone.
Acetanisole is found naturally in castoreum, the glandular secretion of the beaver.
Preparation
Acetanisole can be prepared synthetically by Friedel-Crafts acylation of anisole with acetyl chloride:
Application
It is used as a cigarette additive, a fragrance, and a flavoring in food.
Reactions
4-Methoxyacetophenone is a standard substrate or product of much research, such as transfer hydrogenation and directed arylations.
References
- ^ Para-Acetanisole, The Good Scents Company
- ^ 4'-Methoxyacetophenone from PubChem
- ^ Acetanisole at Sigma-Aldrich
- Tobacco Documents | Profiles | Additives | Acetanisole Archived April 11, 2008, at the Wayback Machine
- 21 CFR 172.515
- Noyori, Ryoji; Yamakawa, Masashi; Hashiguchi, Shohei (2001). "Metal−Ligand Bifunctional Catalysis: A Nonclassical Mechanism for Asymmetric Hydrogen Transfer between Alcohols and Carbonyl Compounds". The Journal of Organic Chemistry. 66 (24): 7931–7944. doi:10.1021/jo010721w. PMID 11722188.
- Palucki, Michael; Buchwald, Stephen L. (1997). "Palladium-Catalyzed α-Arylation of Ketones". Journal of the American Chemical Society. 119 (45): 11108–11109. doi:10.1021/ja972593s.