| Revision as of 05:38, 21 March 2024 editMichael7604 (talk | contribs)Extended confirmed users8,895 editsm Michael7604 moved page Curium hydroxide to Curium(III) hydroxide over redirect← Previous edit | Latest revision as of 10:30, 3 November 2024 edit undoGraeme Bartlett (talk | contribs)Administrators250,235 edits CAS | ||
| Line 9: | Line 9: | ||
| |SystematicName = Curium(3+) oxidanide | |SystematicName = Curium(3+) oxidanide | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| |CASNo = 49848-26-2 | |||
| |CASNo_Ref = {{Cascite|changed|EPA}} | |||
| |PubChem = 14648287 | |PubChem = 14648287 | ||
| |SMILES = ... | |SMILES = ... | ||
| |StdInChI = 1S/Cm.3H2O/h;3*1H2/q+3;;;/p-3 | |StdInChI = 1S/Cm.3H2O/h;3*1H2/q+3;;;/p-3 | ||
| |StdInChI_Ref = {{stdinchicite| |
|StdInChI_Ref = {{stdinchicite|correct|EPA}} | ||
| |StdInChIKey = ZOFUDUXHUCRFKX-UHFFFAOYSA-K | |StdInChIKey = ZOFUDUXHUCRFKX-UHFFFAOYSA-K | ||
| |StdInChIKey_Ref = {{stdinchicite| |
|StdInChIKey_Ref = {{stdinchicite|correct|EPA}} | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
Latest revision as of 10:30, 3 November 2024
 Curium hydroxide | |
| Names | |
|---|---|
| IUPAC name Curium hydroxide | |
| Systematic IUPAC name Curium(3+) oxidanide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CmH3O3 |
| Molar mass | 298 g·mol |
| Appearance | colorless or pale yellow solid |
| Solubility in water | insoluble |
| Structure | |
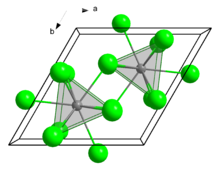
| Crystal structure | hexagonal, UCl3 structure |
| Space group | P63/m, No. 176 |
| Lattice constant | a = 639,1 pm, c = 371,2 pm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |

Curium hydroxide Cm(OH)3 is a radioactive compound first discovered in measurable quantities in 1947. It is composed of a single curium atom and three hydroxy groups. It was the first curium compound ever isolated.
Curium hydroxide is an anhydrous colorless or light-yellow amorphous gelatinous solid that is insoluble in water.
Due to self-irradiation, the crystal structure of Cm(OH)3 decomposes within one day (Cm has a half-life of 18.11 years); for Am(OH)3 the same process takes 4 to 6 months (Am has a half-life of 432.2 years).
See also
References
- ^ Macintyre, Jane E. (1992). Dictionary of Inorganic Compounds. CRC Press. p. 3046. ISBN 978-0-412-30120-9.
- ^ Krivovichev, Sergey; Burns, Peter; Tananaev, Ivan (2006). Structural Chemistry of Inorganic Actinide Compounds. Elsevier. p. 68. ISBN 978-0-08-046791-7.
- Seaborg, Glenn T. (1963). Man-Made Transuranium Elements. Prentice-Hall.
- "WebElements Periodic Table: Curium". webelements.com. Retrieved January 20, 2019.
- Koch, Günter (1972). Transurane Teil C: Die Verbindungen. Gmelins Handbuch (in German). Springer-Verlag. p. 35. ISBN 978-3-662-11547-3.
| Curium compounds | |
|---|---|
| Curium(III) | |
| Curium(IV) | |
| Curium(VI) | |
| Hydroxides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |