| Revision as of 15:21, 24 October 2007 editGreg L (talk | contribs)Extended confirmed users, Pending changes reviewers31,897 edits Vandalims reverted to last version by Slashme← Previous edit | Latest revision as of 00:02, 6 January 2025 edit undoHeyElliott (talk | contribs)Extended confirmed users118,978 edits MOS:REFPUNCTTag: 2017 wikitext editor | ||
| Line 1: | Line 1: | ||
| {{Short description|Metric unit of mass}} | |||
| {{Redirect|Kg}} | |||
| {{Redirect|kg||KG (disambiguation){{!}}KG}} | |||
| ]-] alloy and is stored in a vault at the ] in ], France. For other kilogram-related images, see '']'', below.]]<!--NOTE TO EDITORS: Before changing the picture size, editors are asked to see ] on the discussion page.--> | |||
| {{Use dmy dates|date=October 2024}} | |||
| The '''kilogram''' or '''kilogramme''' (symbol: '''kg''') is the ] ] of ]. The kilogram is defined as being equal to the mass of the ''International Prototype Kilogram'' (IPK), which is almost exactly equal to the mass of one ] of water. It is the only SI base unit with an ] as part of its name. It is also the only SI unit that is still defined in relation to an ] rather than to a fundamental physical property that can be reproduced in different laboratories. | |||
| {{Infobox unit | |||
| | name = kilogram | |||
| While the ] of objects (their gravitational ]) is often given in kilograms, the kilogram is, in the strict scientific sense, a unit of mass. The equivalent unit of force is the non-SI ]. Similarly, the ] ], used in both the ] and ], is a unit of mass and its related unit of force is the ]. The avoirdupois pound is defined as exactly 0.453<font size="-1"> </font>592<font size="-1"> </font>37 kg, making one kilogram approximately equal to 2.205 avoirdupois pounds. | |||
| | image = Poids fonte 5 kg à 2 hg 02.jpg | |||
| | caption = A series of 5, 2, 1, 0.5 and 0.2 kilogram weights, made of ] | |||
| Many units in the SI system are defined relative to the kilogram so its stability is important. After the International Prototype Kilogram had been found to vary in mass over time, the ] (known by the initials CIPM) recommended in 2005 that the kilogram be redefined in terms of fundamental constants of nature.<ref name="94thCIPM">''Proceedings of the 94th meeting (October 2005) of the International Committee for Weights and Measures'', ()</ref> | |||
| | standard = ] | |||
| | quantity = ] | |||
| ==The nature of mass== | |||
| | symbol = kg | |||
| The kilogram is a unit of ], the measurement of which corresponds to the general, everyday notion of how “heavy” something is. However, mass is actually an '']l'' property; that is, the tendency of an object to remain at constant velocity unless acted upon by an outside ]. An object with a mass of one kilogram will ] at ] (about one-tenth the acceleration of Earth’s gravity) when acted upon (pushed by) a force of one ] (symbol: N). | |||
| | units1 = ] | |||
| | inunits1 = {{Ublist|≈ {{val|2.2046}} ]<ref group="Note">The avoirdupois pound is part of both ] and the ]. It is ] {{val|0.45359237|u=kilograms}}.</ref>|≈ {{val|35.274|ul=oz}}}} | |||
| While the '']'' of matter is entirely dependent upon the strength of the local gravitational field, the ''mass'' of matter is constant (assuming it is not traveling at a ] speed with respect to an observer). Accordingly, for astronauts in microgravity, no effort is required to hold objects off the cabin floor since such objects naturally hover; they are “weightless.” However, since objects in microgravity still retain their mass, an astronaut must exert one hundred times more force to ''accelerate'' a 100-kilogram object at the same rate as a 1-kilogram object. See also '']'', below. | |||
| | units2 = British Gravitational | |||
| | inunits2 = ≈ {{val|0.0685}} ] | |||
| == SI multiples == | |||
| | units3 = ]s | |||
| <!--NOTE TO EDITORS: This section is linked from ] as well as many other Misplaced Pages articles. Please do not delete or rename. | |||
| | inunits3 = {{val|1000|ul=g}} | |||
| | units4 = Daltons | |||
| --> | |||
| | inunits4 = {{val|6.02214076|e=26|ul=Da}} | |||
| Because ] may not be ]d within the name or symbol for a unit of measure, SI prefixes are used with the ''],'' not the kilogram, which already has a prefix as part of its name.<ref>NIST: ''SI prefixes'' ().</ref> For instance, one-millionth of a kilogram is 1 mg (one milligram), not 1 µkg (one microkilogram). | |||
| <div style="float:center; margin-left: 1em;"> | |||
| {{SI multiples | |||
| |unit=gram | |||
| |symbol=g | |||
| |note=Common prefixes are in bold face.<ref>Criterion: A combined total of at least 250,000 Google hits on both the U.S. spelling (-gram) and the U.K./International spelling (-gramme).</ref> | |||
| |p= | n= | mc= | m= | k= | |||
| |xM = megagram (]) | |||
| |xmc = microgram (mcg) | |||
| }} | }} | ||
| </div> | |||
| <br> | |||
| * When the Greek lowercase “µ” (mu) in the symbol of microgram is typographically unavailable, it is occasionally—although not properly—replaced by Latin lowercase “u”. | |||
| * The microgram is often abbreviated “mcg”, particularly in pharmaceutical and nutritional supplement labeling, to avoid confusion since the “µ” prefix is not well recognized outside of technical disciplines.<ref>The practice of using the abbreviation “mcg” rather than the SI symbol “µg” was formally mandated for medical practitioners in 2004 by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) in their because hand-writen expressions of “µg” can be confused with “mg”, resulting in a thousand-fold overdosing. The mandate was also adopted by the </ref> Note however, that the ''abbreviation'' “mcg”, is also the ''symbol'' for an obsolete ] unit of measure known as the “millicentigram,” which is equal to 10 µg. | |||
| * The unit name “megagram” is rarely used, and even then, typically only in technical fields in contexts where especially rigorous consistency with the units of measure is desired. For most purposes, the term “],” or “metric ton” is instead used. | |||
| The '''kilogram''' (also spelled '''kilogramme'''<ref name=":1" />) is the ] of ] in the ] (SI), having the unit symbol '''kg'''.<ref name=":1" /> 'Kilogram' means 'one thousand ]s'<ref>{{Cite web |title=Kilogram |url=https://www.collinsdictionary.com/dictionary/english/kilogram |access-date=14 October 2024 |website=Collins Online Dictionary}}</ref> and is colloquially abbreviated to '''kilo'''.<ref></ref> | |||
| == History == | |||
| ====Early definitions==== | |||
| :''See also ] for more on the history of the kilogram.'' | |||
| The kilogram is an SI ], defined ultimately in terms of three ]s of the SI, namely ] of the ] atom, the ], and the ].<ref name="SIBrochure9thEd"/>{{rp|131}} A properly equipped ] laboratory can calibrate a mass measurement instrument such as a ] as a primary standard for the kilogram mass.<ref>{{Cite web |date=July 7, 2021 |title=Mise en pratique for the definition of the kilogram in the SI |url=https://www.bipm.org/documents/20126/41489673/SI-App2-kilogram.pdf/5881b6b5-668d-5d2b-f12a-0ef8ca437176?version=1.9&t=1637237674882&download=false |access-date=February 18, 2022 |website=BIPM.org}}</ref> | |||
| On 7 April 1795, the ] was decreed in ] to be equal to “the absolute weight of a volume of pure water equal to a cube of one hundredth of a meter, and to the temperature of the melting ice.”<ref>''''</ref> The regulation of trade and commerce required a practical reference standard in addition to the definition based on fundamental physical properties. Accordingly, a provisional kilogram standard was made as a single-piece, metallic reference standard one thousand times more massive than the gram. | |||
| The kilogram was originally defined in 1795 during the ] as the mass of one ] of ]. The current definition of a kilogram agrees with this original definition to within 30 ]. In 1799, the platinum '']'' replaced it as the standard of mass. In 1889, a cylinder composed of ], the ] (IPK), became the standard of the unit of mass for the metric system and remained so for 130 years, before the current standard was ].<ref name="vox" /> | |||
| In addition to this provisional kilogram standard, work was commissioned to determine precisely how massive a cubic decimeter (now defined as one ]) of water is. Although the decreed definition of the kilogram specified water at 0 °C — a highly stable ''temperature'' point — the scientists chose to redefine the standard and perform their measurements at the most stable ''density'' point: the temperature at which water reaches maximum density, which was measured at the time as 4 °C.<ref>Citation: ''L'Histoire Du Mètre, La Détermination De L'Unité De Poids'', link to Web site </ref> They concluded that one cubic decimeter of water at its maximum density was equal to 99.92072% of the mass of the provisional kilogram made earlier that year.<ref>Citation: ''''</ref> Four years later in 1799, an all-platinum ] kilogram, the ''Kilogramme des Archive'' (Kilogram of the Archives), was fabricated with the objective that it would equal, as close as was scientifically feasible for the day, the mass of a cubic decimeter of water at 4 °C. The kilogram was defined to be equal to the mass of the Kilogram of the Archives and this standard stood for the next ninety years. | |||
| == Definition == | |||
| ====International Prototype Kilogram==== | |||
| The kilogram is defined in terms of three defining constants:<ref name="SIBrochure9thEd">{{citation |title=The International System of Units (SI) |author=International Bureau of Weights and Measures |author-link=New SI |date=20 May 2019 |edition=9th |isbn=978-92-822-2272-0 |url=https://www.bipm.org/utils/common/pdf/si-brochure/SI-Brochure-9.pdf| archive-url = https://web.archive.org/web/20211018184555/https://www.bipm.org/documents/20126/41483022/SI-Brochure-9.pdf/fcf090b2-04e6-88cc-1149-c3e029ad8232 |archive-date=18 October 2021 |url-status=live}}</ref> | |||
| <!--NOTE TO EDITORS: This section is externally linked to from ]. Please do not delete or rename without fixing the referencing link.--> | |||
| * a specific atomic transition frequency {{math|]}}, which defines the duration of the second, | |||
| Since 1889, the ] system defines the ] of the kilogram to be equal to the mass of the '''International Prototype Kilogram''' — often referred to in the professional ] world as the “'''IPK'''”. The IPK comprises an ] of 90% ] and 10% ] (by weight) and is machined into a right-circular cylinder (height = diameter) of 39.17 ] to minimize its surface area.<ref name="Quinn">''New Techniques in the Manufacture of Platinum-Iridium Mass Standards'', T. J. Quinn, Platinum Metals Rev., 1986, '''30''', (2), Pg. 74 – 79</ref> The IPK and six of its official copies (its “sister copies”) are stored in an environmentally monitored vault in the basement of the ] House of Breteuil in ] on the outskirts of ] (see '']'', below for images). Three independently controlled keys are required to open the vault. Official copies of the IPK were made available to other nations to serve as their national standards. These are compared to the IPK roughly every 40 years. | |||
| * the ] {{mvar|c}}, which when combined with the second, defines the length of the metre, | |||
| * and the ] {{mvar|h}}, which when combined with the metre and second, defines the mass of the kilogram. | |||
| The formal definition according to the ] (CGPM) is: | |||
| <!-- this is an exact quote. Do not change it.-->{{Blockquote|The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the ] {{mvar|h}} to be {{val|6.62607015|e=-34}} when expressed in the unit J⋅s, which is equal to kg⋅m<sup>2</sup>⋅s<sup>−1</sup>, where the ] and the ] are defined in terms of {{mvar|c}} and {{math|Δ''ν''<sub>Cs</sub>}}.|source= CGPM<ref name="draft-resolution-A"> | |||
| {{citation | |||
| |title=Draft Resolution A "On the revision of the International System of units (SI)" to be submitted to the CGPM at its 26th meeting (2018) | |||
| |url=https://www.bipm.org/utils/en/pdf/CGPM/Draft-Resolution-A-EN.pdf | |||
| |archive-url=https://web.archive.org/web/20210402142630/https://www.bipm.org/utils/en/pdf/CGPM/Draft-Resolution-A-EN.pdf | |||
| |archive-date=April 2, 2021 | |||
| |url-status=live | |||
| }}</ref><ref>. The day is the 144th anniversary of the ].</ref>}} | |||
| Defined in term of those units, the kg is formulated as:<ref>. BIPM, 9th edition, 2019.</ref> | |||
| {{block indent|1=kg = {{math|{{sfrac|({{val|299792458}}){{sup|2}}|({{val|6.62607015|e=-34}})({{val|9192631770}})}}{{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''c''{{sup|2}}}}}}}} | |||
| The IPK is one of three cylinders made in 1879. In 1883, it was found to be indistinguishable from the mass of the Kilogram of the Archives made eighty-four years prior, and was formally ratified as ''the'' kilogram by the 1st ] in 1889.<ref name="Quinn"/> Modern measurements of the density of purified water that has a carefully controlled isotopic composition (known as ]) show that a cubic decimeter (one liter) of water at its point of maximum density, 3.984 °C, has a mass that is 25.05 parts per million less than the kilogram.<ref>''Water Structure and Science, Water Properties, Density maximum (and molar volume) at temperature of maximum density, a'' (by London South Bank University). </ref> This small, 25 ppm difference, and the fact that the mass of the IPK was indistinguishable from the mass of the Kilogram of the Archives, speak volumes of the scientists’ skills over {{age|1799|1|1}} years ago when making their measurements of water’s properties and in manufacturing the Kilogram of the Archives. | |||
| {{block indent|1={{0|kg }}≈ {{math|({{val|1.475521399735270|e=40}}){{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''c''{{sup|2}}}}}}.}} | |||
| This definition is generally consistent with previous definitions: the ] remains within 30 ] of the mass of one litre of water.<ref>The density of water is {{val|0.999972|u=g/cm3}} at {{val|3.984|u=°C}}. See {{cite book |last=Franks |first=Felix |title=The Physics and Physical Chemistry of Water |url=https://books.google.com/books?id=5f_xBwAAQBAJ&pg=PA376 |year=2012 |publisher=Springer |isbn=978-1-4684-8334-5}}</ref> | |||
| ==Stability of the International Prototype Kilogram== | |||
| By definition, the error in the measured value of the IPK’s mass is exactly zero; the IPK ''is'' the kilogram. However, any changes in the IPK’s mass over time can be deduced by comparing its mass to that of its official copies stored throughout the world, a process called “periodic verification.” For instance, the U.S. owns three kilogram ], two of which, K4 and K20, are from the original batch of 40 replicas of the IPK delivered in 1884. The K20 replica was designated as the ] of mass for the U.S. Both of these, as well as those from other nations, are periodically returned to the BIPM for verification.<ref>Extraordinary care is exercised when transporting prototypes. In 1984, the K4 and K20 replicas were hand-carried in the passenger section of a commercial airliner.</ref> | |||
| === Timeline of previous definitions === | |||
| Note that the masses of the replicas are not precisely equal to that of the IPK; their masses are calibrated and documented as offset values. For instance, K20, the U.S.’s primary standard, originally had an official mass of 1 kg – 39 µg in 1889; that is to say, K20 was 39 µg less than the IPK. A verification performed in 1948 showed a mass of 1 kg – 19 µg. The latest verification performed in 1999 shows a mass identical to its original 1889 value. The mass of K4, the U.S.’s ], as of 1999 was officially calibrated as 1 kg – 116 µg. However, it was 41 µg more massive (in comparison to the IPK) in 1889. | |||
| ], whose mass was defined to be one kilogram from 1889 to 2019.]] | |||
| * 1793: The ] (the precursor of the kilogram) was defined as the mass of 1 ] (dm<sup>3</sup>) of water, which was determined to be 18841 ].<ref>{{cite book |title=Annales de chimie ou Recueil de mémoires concernant la chimie et les arts qui en dépendent |date=1792|url=https://books.google.com/books?id=FufDNJHvgFEC&q=18841+grains+grave&pg=RA1-PA278 |location= Paris |publisher= Chez Joseph de Boffe |page= 277 |volume = 15–16| last1=Guyton| last2=Lavoisier| last3=Monge| last4=Berthollet| display-authors=etal|author-link1=Louis-Bernard Guyton de Morveau| author-link2=Antoine Lavoisier| author-link3=Gaspard Monge| author-link4=Claude Louis Berthollet}}</ref> | |||
| * 1795: the gram (<sup>1</sup>/<sub>1000</sub> of a kilogram) was provisionally defined as the mass of one cubic ] of water at the melting point of ice.<ref>{{lang|fr|Gramme, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante}}</ref> | |||
| * 1799: The ] was manufactured as a prototype. It had a mass equal to the mass of 1 dm<sup>3</sup> of water at the temperature of its maximum density, which is approximately 4 ].<ref name="Zupko">{{cite book |last=Zupko |first=Ronald Edward| author-link =Ronald Edward Zupko|date=1990 |title=Revolution in Measurement: Western European Weights and Measures Since the Age of Science |url=https://archive.org/details/bub_gb_uYCNFkRgXCoC |location=Philadelphia |publisher=American Philosophical Society |isbn=978-0-87169-186-6}}</ref> | |||
| * 1875–1889: The ] was signed in 1875, leading to the production of the ] (IPK) in 1879 and its adoption in 1889.<ref>{{cite encyclopedia|url = http://www.britannica.com/EBchecked/topic/378767/Treaty-of-the-Metre|title = Treaty of the Metre|encyclopedia = ]|access-date = 18 July 2023|year = 2023}}</ref> | |||
| * 2019: The kilogram was ] in terms of the ], the ] and ] as approved by the ] (CGPM) on 16 November 2018.<ref name="vox"/> | |||
| == Name and terminology == | |||
| Since the IPK and its replicas are stored in air (albeit under two or more nested bell jars), they adsorb atmospheric contamination onto their surfaces and gain mass. Accordingly, they are cleaned in preparation for periodic verifications—a process the BIPM developed between 1939 and 1946 known as “the BIPM cleaning method” that includes steam cleaning, lightly rubbing with chemical-soaked ], and allowing the ] to settle for 7–10 days. Cleaning the prototypes removes between 5 and 60 µg of contamination depending largely on the time elapsed since the last cleaning. Further, a second cleaning can remove up to 10 µg more. After cleaning—even when they are stored in their bell jars—the IPK and its replicas immediately begin gaining mass again. The BIPM even developed a model of this gain and concluded that it averaged 1.11 µg per month for the first 3 months after cleaning and then decreased to an average of about 1 µg per year thereafter. Since check standards like K4 are not cleaned for routine calibrations of other mass standards—a precaution to minimize the potential for wear and handling damage—the BIPM’s model has been used as an “after cleaning” correction factor. | |||
| The kilogram is the only ] with an ] (''kilo'') as part of its name. The word ''kilogramme'' or ''kilogram'' is derived from the ] {{lang|fr|kilogramme}},<ref name=OED/> which itself was a learned coinage, prefixing the ] stem of {{lang|grc|χίλιοι}} {{transl|grc|khilioi}} "a thousand" to {{lang|la|gramma}}, a Late Latin term for "a small weight", itself from Greek {{lang|grc|γράμμα}}.<ref> | |||
| {{cite book | |||
| |title = The Concise Oxford Dictionary | |||
| |year = 1964 | |||
| |last1 = Fowlers |first1 = HW | |||
| |last2 = Fowler |first2 = FG | |||
| |publisher = The Clarendon Press | |||
| |location = Oxford | |||
| }} Greek {{lang|grc|γράμμα}} (as it were {{lang|grc|]-]}}, Doric {{lang|grc|γράθμα}}) means "something written, a letter", but it came to be used as a unit of weight, apparently equal to {{sfrac|1|24}} of an ] ({{sfrac|1|288}} of a {{lang|la|]}}, which would correspond to about 1.14 grams in modern units), at some time during Late Antiquity. French {{lang|fr|gramme}} was adopted from Latin {{lang|la|gramma}}, itself quite obscure, but found in the {{lang|la|Carmen de ponderibus et mensuris}} (8.25) attributed by ] (fl. 1st century), where it is the weight of two {{lang|la|]}} (Charlton T. Lewis, Charles Short, ''A Latin Dictionary'' , 1879). | |||
| Henry George Liddell. Robert Scott. '']'' (revised and augmented edition, Oxford, 1940) , citing the 10th-century work '']'' and a 4th-century papyrus edited in L. Mitteis, ''Griechische Urkunden der Papyrussammlung zu Leipzig'', vol. i (1906), 62 ii 27.</ref> | |||
| The word {{lang|fr|kilogramme}} was written into French law in 1795, in the ''Decree of ]'',<ref> | |||
| {{cite web | |||
| |url = http://mjp.univ-perp.fr/france/1793mesures.htm | |||
| |title = Décret relatif aux poids et aux mesures du 18 germinal an 3 (7 avril 1795) | |||
| |language = fr | |||
| |trans-title=Decree of 18 Germinal, year III (April 7, 1795) regarding weights and measures | |||
| |website = Grandes lois de la République | |||
| |publisher = Digithèque de matériaux juridiques et politiques, Université de Perpignan | |||
| |access-date = November 3, 2011 | |||
| }}</ref> | |||
| which revised the provisional system of units introduced by the French ] two years earlier, where the {{lang|fr|gravet}} had been defined as weight ({{lang|fr|poids}}) of a cubic centimetre of water, equal to 1/1000 of a {{lang|fr|]}}.<ref>{{lang|fr|Convention nationale, décret du 1<sup>er</sup> août 1793, ed. Duvergier, ''Collection complète des lois, décrets, ordonnances, règlemens avis du Conseil d'état, publiée sur les éditions officielles du Louvre''|italic=unset}}, vol. 6 (2nd ed. 1834), . | |||
| The ''metre'' ({{lang|fr|mètre}}) on which this definition depends was itself defined as the ten-millionth part of a quarter of Earth's ], given in ] as 3 {{lang|fr|]}}, 11.44 {{lang|fr|lignes}} (a {{lang|fr|ligne}} being the 12th part of a {{lang|fr|pouce}} (inch), or the 144th part of a {{lang|fr|pied}}.</ref> In the decree of 1795, the term {{lang|fr|gramme}} thus replaced {{lang|fr|gravet}}, and {{lang|fr|kilogramme}} replaced {{lang|fr|grave}}.<ref name="Zupko"/> | |||
| The French spelling was adopted in Great Britain when the word was used for the first time in English in 1795,<ref> | |||
| Because the first forty official copies are made of precisely the same alloy as the IPK and are stored under similar conditions, periodic verifications using a large number of replicas—especially the national primary standards, which are rarely used—can convincingly demonstrate the stability of the IPK. What has become clear after the third periodic verification performed between 1988 and 1992 is that the mass of the IPK lost perhaps 50 µg over the last century, and possibly significantly more, in comparison to its official copies.<ref name="Redef">''Redefinition of the kilogram: a decision whose time has come'', Ian M. Mills ''et al.'', Metrologia '''42''' (2005), 71–80</ref><ref>''The Third Periodic Verification of National Prototypes of the Kilogram (1988–1992)'', G. Girard, Metrologia '''31''' (1994) 317–336</ref> The answer as to why this might be the case has proved elusive for physicists who have dedicated their careers to the SI unit of mass. No plausible mechanism has been proposed to explain either a steady decrease in the mass of the IPK, or an increase in that of its six sister copies and the others dispersed throughout the world.<ref>''The SI unit of mass'', Richard Davis, Metrologia '''40''' (2003), 299–305. Note that if the ∆50 µg between the IPK and its replicas was entirely due to wear, the IPK would have to have lost 150 million billion more platinum and iridium atoms over the last century than its replicas. That there would be this much wear, much less a ''difference'' of this magnitude, is thought unlikely; 50 µg is roughly the mass of a fingerprint. Many theories have been advanced to explain the data, including one that begins with the observation that the IPK is uniquely stored under three bell jars whereas its six sister copies and the others dispersed throughout the world are stored under only two. This theory is founded on two other facts: that platinum has a strong affinity for mercury, and that atmospheric mercury is significantly more abundant in the atmosphere today than at the time the IPK and its replicas were manufactured. This theory posits that the relative change in mass between the IPK and its replicas is not one of loss at all, and is instead a simple matter that the IPK has ''gained less'' than the replicas. This theory is just one of many advanced by the specialists to account for the relative change in mass. To date, each theory has either proven implausible, or there is insufficient data or technical means to either prove or disprove it. Citation: ''Conjecture why the IPK drifts'', R. Steiner, NIST, 11 Sept. 2007.</ref> Further, the IPK exhibits an instability of about 30 µg over a period of about a month in its after-cleaned mass.<ref>Report to the CGPM, 14th meeting of the Consultative Committee for Units (CCU), April 2001, 2. (ii); ''General Conference on Weights and Measures, 22nd Meeting, October 2003'', which stated “The kilogram is in need of a new definition because the mass of the prototype is known to vary by several parts in 10<sup>8</sup> over periods of time of the order of a month…” ().</ref> The precise reason for this instability is not fully understood but is thought to entail surface effects: microscopic differences in their polished surfaces, unintentional differences in the cleaning process, and/or differences in the precise nature of the contamination. What ''is'' known is the past assumption that the cleaning process reliably restores the prototypes to their original value is false and the BIPM’s after-cleaning correction factor is useful only for long-term trends. Scientists are seeing far greater variability in the prototypes than previously believed. Further, there is no technical means available to know whether or not the entire worldwide ensemble of prototypes suffer from even greater long-term trends upwards or downwards because their mass “relative to an invariant of nature is unknown at a level below 1000 µg over a period of 100 or even 50 years.”<ref name="Redef"/><br><br><!-- | |||
| {{cite journal | |||
| |url = https://books.google.com/books?id=24QCAAAAYAAJ&q=kilogramme+weights&pg=PA557 | |||
| |title = Paris, during the year 1795 | |||
| |author = Peltier, Jean-Gabriel | |||
| |journal = Monthly Review |date=1795 |volume=17|pages=556|access-date = August 2, 2018 | |||
| }} Contemporaneous English translation of the French decree of 1795</ref><ref name=OED> | |||
| {{cite web | |||
| |url = http://www.oed.com/viewdictionaryentry:showfullentry/true?t:ac=Entry/103396 | |||
| |website = Oxford English Dictionary | |||
| |publisher = Oxford University Press | |||
| |title = Kilogram | |||
| |access-date = November 3, 2011 | |||
| }}{{Dead link|date=August 2024 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> with the spelling ''kilogram'' being adopted in the United States. In the United Kingdom both spellings are used, with "kilogram" having become by far the more common.<ref name=":1"> | |||
| {{cite web | |||
| |url = http://english.oxforddictionaries.com/definition/kilogram | |||
| |title = Kilogram | |||
| |website = Oxford Dictionaries | |||
| |access-date = November 3, 2011 | |||
| |url-status = dead | |||
| |archive-url = https://archive.today/20130131014115/http://english.oxforddictionaries.com/definition/kilogram | |||
| |archive-date = January 31, 2013 | |||
| |df = mdy-all | |||
| }}</ref> UK law regulating the units to be used when ] does not prevent the use of either spelling.<ref> | |||
| {{cite web | |||
| |url = http://www.legislation.gov.uk/ukpga/1985/72/section/92 | |||
| |title = Spelling of "gram", etc | |||
| |website = ] | |||
| |publisher = ] | |||
| |date = October 30, 1985 | |||
| |access-date = November 6, 2011 | |||
| }}</ref> | |||
| In the 19th century the French word {{lang|fr|kilo}}, a ] of {{lang|fr|kilogramme}}, was imported into the English language where it has been used to mean both kilogram<ref> | |||
| -->The relative change in mass and the instability in the IPK has prompted research into improved methods to obtain a smooth surface finish using diamond-turning on newly manufactured replicas and has intensified the search for a new definition of the kilogram. See '']'', below.<ref>General section citations: ''Recalibration of the U.S. National Prototype Kilogram'', R. S. Davis, Journal of Research of the National Bureau of Standards, '''90''', No. 4, July–August 1985 (); and ''The Kilogram and Measurements of Mass and Force'', Z. J. Jabbour ''et al.'', J. Res. Natl. Inst. Stand. Technol. '''106''', 2001, 25–46 ()</ref> | |||
| {{cite encyclopedia | |||
| |year=1989 | |||
| |edition = 2nd | |||
| |title = kilo (n1) | |||
| |encyclopedia = ] | |||
| |publisher = Oxford University Press | |||
| |location = Oxford | |||
| |url = http://www.oed.com/viewdictionaryentry/Entry/103394 | |||
| |access-date = November 8, 2011}}</ref> and kilometre.<ref>{{cite encyclopedia | |||
| |year = 1989 | |||
| |edition = 2nd | |||
| |title = kilo (n2) | |||
| |encyclopedia = ] | |||
| |publisher = Oxford University Press | |||
| |location = Oxford | |||
| |url = http://www.oed.com/viewdictionaryentry/Entry/103395 | |||
| |access-date = November 8, 2011 | |||
| }}</ref> While ''kilo'' as an alternative is acceptable, to '']'' for example,<ref>{{cite news |url=http://www.frzee.com/Education/The%20Economist%20Style%20Guide.pdf |title=Style Guide |newspaper=] |date=January 7, 2002 |archive-url=https://web.archive.org/web/20170701053545/http://www.frzee.com/Education/The%20Economist%20Style%20Guide.pdf |archive-date=July 1, 2017 |url-status=dead |access-date=November 8, 2011}}</ref> the Canadian government's ] system states that "SI (International System of Units) usage, followed in scientific and technical writing" does not allow its usage and it is described as "a common informal name" on Russ Rowlett's Dictionary of Units of Measurement.<ref> | |||
| {{cite web |url=https://www.btb.termiumplus.gc.ca/tpv2guides/guides/wrtps/index-eng.html?lang=eng&lettr=indx_catlog_k&page=96vUJlKx4UCA.html |website=Termium Plus |publisher=Government of Canada |title=kilogram, kg, kilo |date=October 8, 2009 |access-date =May 29, 2019 }}</ref><ref> | |||
| {{cite web |url=http://www.unc.edu/~rowlett/units/dictK.html |title=kilo |website=How Many? |access-date=November 6, 2011 |url-status=dead |archive-url=https://web.archive.org/web/20111116205434/http://www.unc.edu/~rowlett/units/dictK.html |archive-date=November 16, 2011}}</ref> When the ] gave the metric system legal status in 1866, it permitted the use of the word ''kilo'' as an alternative to the word ''kilogram'',<ref> | |||
| {{cite web | |||
| |url=http://lamar.colostate.edu/~hillger/laws/metric-act-bill.html | |||
| |title=H.R. 596, An Act to authorize the use of the metric system of weights and measures | |||
| |author=29th Congress of the United States, Session 1 | |||
| |date=May 13, 1866 | |||
| |url-status=dead | |||
| |archive-url=https://web.archive.org/web/20150705015307/http://lamar.colostate.edu/~hillger/laws/metric-act-bill.html | |||
| |archive-date=July 5, 2015 | |||
| }}</ref> but in 1990 revoked the status of the word ''kilo''.<ref> | |||
| {{cite journal | |||
| |journal = ] | |||
| |volume = 63 | |||
| |issue = 144 | |||
| |date = July 28, 1998 | |||
| |page = 40340 | |||
| |url = http://physics.nist.gov/cuu/pdf/SIFedReg.pdf | |||
| |title = Metric System of Measurement:Interpretation of the International System of Units for the United States; Notice | |||
| |quote = '''Obsolete Units''' As stated in the 1990 Federal Register notice, ... | |||
| |access-date = November 10, 2011 | |||
| |url-status = dead | |||
| |archive-url = https://web.archive.org/web/20111015081850/http://physics.nist.gov/cuu/pdf/SIFedReg.pdf | |||
| |archive-date = October 15, 2011 | |||
| |df = mdy-all | |||
| }}</ref> | |||
| The SI system was introduced in 1960 and in 1970 the ] started publishing the ], which contains all relevant decisions and recommendations by the ] concerning units. The ''SI Brochure'' states that "It is not permissible to use abbreviations for unit symbols or unit names ...".<ref>{{SIBrochure8th|page = 130}}</ref><ref group = Note>The French text (which is the authoritative text) states "{{lang|fr|Il n'est pas autorisé d'utiliser des abréviations pour les symboles et noms d'unités ...}}"</ref> | |||
| ==Importance of the kilogram== | |||
| As the SI system of measurement is currently defined and structured, the stability of the kilogram is ''crucial'' since it effectively underpins the entire system. For instance, the ]—the SI unit of force—is defined as the force necessary to accelerate the kilogram by one meter per second². Accordingly, if the mass of the IPK were to change slightly, so too must the newton by a proportional degree so the acceleration remains at precisely one meter/second². In turn, the ]—the unit of pressure—is defined in terms of the newton. This chain of dependency follows to all the electrical units. For instance, the ], which is the electrical and mechanical unit of energy, is defined as the energy expended when a force of one newton acts through one ]. The ] too is defined relative to the kilogram. With two of the primary units of electricity thus defined in terms of the kilogram, so too follow all the rest, including the ], ], ], ], ], and ]. | |||
| For use with east Asian character sets, the SI symbol is encoded as a single Unicode character, {{unichar|338f|SQUARE KG}} in the ] block. | |||
| Clearly, having the ] of many of the units comprising the SI system of measurement ultimately defined by the mass of a {{age|1879|1|1}}-year-old, ]-size piece of metal is a tenuous state of affairs. The quality of the IPK must be fanatically protected in order to preserve the integrity of the SI system. Fortunately, '']'' of the SI units are quite different from their ''].'' For instance, the ] is ''defined'' as the distance light travels in a vacuum during a time interval of 1/299,792,458 of a second. However, the meter’s ''practical realization'' typically takes the form of a helium-neon laser, and the meter’s length is '']''—not defined—as 1,579,800.298<font size="-1"> </font>728 wavelengths of light from this laser. Note that the redefinition of the meter in terms of a duration of one second reduced the uncertainty in the wavelength of the laser light. Now suppose that the official measurement of the second was found to have drifted a few parts per billion (it’s actually exquisitely stable). There would be no automatic effect on many of the SI units of measurement because, as with the meter, the duration of the second is often abstracted through other physical principles underlying their practical realizations. Scientists performing meter calibrations would simply continue to measure out the same number of laser wavelengths until an agreement was reached to do otherwise. The same is true with regard to the real-world dependency on the kilogram: if the mass of the IPK was found to have changed slightly, there would be no automatic effect upon the other units of measure because their practical realizations provide an insulating layer of abstraction. Any discrepancy would eventually have to be reconciled though because the virtue of the SI system is its precise mathematical and logical harmony amongst its units. If the IPK’s value ''was'' found to have changed, one quick fix would be to simply redefine the kilogram as being equal to the IPK plus an offset value, similarly to what is currently done with its replicas; e.g., “the kilogram is equal to the mass of the IPK + 42 µg.” | |||
| == Redefinition based on fundamental constants == | |||
| The long-term solution is to liberate the SI system’s dependency on the IPK by developing a practical realization of the kilogram that can be reproduced in different laboratories by following a written specification. The units of measure in such a practical realization would have their magnitudes precisely defined and expressed in terms of fundamental physical constants. While major portions of the SI system would still be based upon the kilogram, the kilogram would in turn be based upon invariant, universal constants of nature. While this is a worthwhile objective and much work towards that end is ongoing, no alternative to date has achieved the uncertainty of a few parts in 10<sup>8</sup> (~30 µg) required to compete with the IPK. The most promising contender, an implementation of the ] by the ] (NIST), as of late 2007 was approaching the level where scientists could ''resolve'' a difference of about 25 µg. See '']'', below. | |||
| ] after the 2019 redefinition: the kilogram is now fixed in terms of the ], the ] and the ]; furthermore the ] no longer depends on the kilogram]] | |||
| ], which was originally used to measure the ] in terms of the IPK, can now be used to calibrate secondary standard weights for practical use.]] | |||
| {{main|2019 revision of the SI}} | |||
| The replacement of the ] (IPK) as the primary standard was motivated by evidence accumulated over a long period of time that the mass of the IPK and its replicas had been changing; the IPK had diverged from its replicas by approximately 50 micrograms since their manufacture late in the 19th century. This led to ] to develop measurement technology precise enough to warrant replacing the kilogram artefact with a definition based directly on physical fundamental constants.<ref name="vox">{{cite news |last1=Resnick |first1=Brian |title=The new kilogram just debuted. It's a massive achievement. |url=https://www.vox.com/science-and-health/2019/5/17/18627757/kilogram-redefined-world-metrology-day-explained |access-date=May 23, 2019 |publisher=vox.com |date=May 20, 2019}}</ref> | |||
| ==Mass vs. weight== | |||
| ====The distinction between the two==== | |||
| ]<!-- | |||
| The ] (CIPM) approved a ] in November 2018 that defines the kilogram by defining the ] to be exactly {{val|6.62607015|e=−34|u=kg⋅m<sup>2</sup>⋅s<sup>−1</sup>}}, effectively defining the kilogram in terms of the second and the metre. The new definition took effect on 20 May 2019.<ref name="vox"/><ref name=draft-resolution-A /><ref> | |||
| --> | |||
| {{cite news | |||
| As stated above in '']'', the kilogram is a unit of mass, which is an inertial property. Inertia is the property that is sensed when pushing horizontally to accelerate a ] that is resting on a level, smooth surface. This is quite distinct from “weight,” which is the downwards gravitational force of the bowling ball that one must counter when holding it off the floor. Unless ] effects apply, mass is an unchanging, universal property of matter that is unaffected by gravity. ''Weight'' on the other hand, is a property of matter that is entirely dependent upon the local strength of gravity. For instance, an astronaut’s weight on the Moon is one-sixth of that on the Earth, whereas his mass has changed little during the trip. Consequently, wherever the physics of ''recoil kinetics'' (mass, velocity, inertia, ] and ]s) dominate and the influence of gravity is a negligible factor, the behavior of objects remains consistent even where gravity is relatively weak. For instance, billiard balls on a billiards table would scatter and recoil with the same speeds and energies after a break shot on the Moon as on Earth; they would however, drop into the pockets much more slowly. | |||
| |url= https://www.bbc.com/news/science-environment-46143399 | |||
| |title= Kilogram gets a new definition | |||
| |author= Pallab Ghosh | |||
| |date= November 16, 2018 | |||
| |journal= BBC News | |||
| |access-date= November 16, 2018 | |||
| }}</ref> | |||
| Prior to the redefinition, the kilogram and several other SI units based on the kilogram were defined by a man-made metal artifact: the '']'' from 1799 to 1889, and the IPK from 1889 to 2019.<ref name="vox"/> | |||
| In the ]s, the terms “]” and “]” are rigidly defined as separate measures in order to enforce clarity and precision. In everyday use, given that all masses on Earth have weight and this relationship is usually highly proportional,<ref>On Earth, masses with densities less than that of air float and have ''negative weight;'' that is, they are ]. Such masses have positive weight in a vacuum.</ref> “weight” often serves to describe both properties, its meaning being dependent upon context. For example, the “net weight” of retail products, which may be given in pounds (U.S.) and kilograms, refers to mass (see also ''])''. Conversely, the “]” rating on automobile tires, which specifies the maximum ] for a tire in kilograms, actually refers to weight; that is, the force due to gravity. | |||
| In 1960, the ], previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by ],<ref>{{SIbrochure8th|page=112}}</ref> and later the ]) so that the standard can be independently reproduced in different laboratories by following a written specification. | |||
| ====The unit of force: kilogram-force==== | |||
| When an object’s weight (its gravitational force) is expressed in kilograms, the unit of measure is not a true kilogram; it is the ] (kgf or kg-f), also known as the ''kilopond'' (kp), which is a non-SI unit of force. All objects on Earth are subject to a gravitational acceleration of approximately 9.8 m/s². The ] (also known as the “General Conference on Weights and Measures”) fixed the value of ] at precisely 9.80665 m/s² so that disciplines such as ] would have a standard value for converting units of defined mass into defined forces and ]s. In fact, the kilogram-force is defined as precisely 9.80665 newtons. As a practical matter, gravitational acceleration (symbol: ''g)'' varies slightly with ], ] and subsurface density; these variations are typically only a few tenths of a percent. See also '']''. | |||
| At the 94th Meeting of the CIPM in 2005, it was recommended that the same be done with the kilogram.<ref> | |||
| Since masses are rarely measured to an ] of better than one percent, it is technically just as valid to state that a one-kilogram object on Earth has a ''weight'' of one kilogram-force as it is to state that it has a ''mass'' of one kilogram. Accordingly, it may correctly be assumed that when someone speaks or writes of a “weight” in kilograms, they are referring to the gravitational load of the kilogram and the proper “kilogram-''force”'' is implied. | |||
| {{cite conference | |||
| |url=https://www.bipm.org/cc/CIPM/Allowed/94/CIPM-Recom1CI-2005-EN.pdf | |||
| |conference=94th meeting of the International Committee for Weights and Measures | |||
| |title=Recommendation 1: Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants | |||
| |date=October 2005 | |||
| |page=233 | |||
| |access-date=February 7, 2018 | |||
| |url-status=live | |||
| |archive-url=https://web.archive.org/web/20070630011658/https://www.bipm.org/cc/CIPM/Allowed/94/CIPM-Recom1CI-2005-EN.pdf | |||
| |archive-date=June 30, 2007 | |||
| }}</ref><!-- Original URL: http://www.bipm.org/utils/en/pdf/CIPM2005-EN.pdf --> | |||
| In October 2010, the CIPM voted to submit a resolution for consideration at the ] (CGPM), to "take note of an intention" that the kilogram be defined in terms of the ], {{math|''h''}} (which has dimensions of energy times time, thus mass × length{{sup|2}} / time) together with other physical constants.<ref>{{cite journal|url=https://www.nist.gov/pml/wmd/20101026_si.cfm |title=NIST Backs Proposal for a Revamped System of Measurement Units |journal=NIST |date=October 26, 2010 |publisher=Nist.gov |access-date=April 3, 2011}}</ref><ref name="draft"> | |||
| ====Converting mass to weight in engineering==== | |||
| {{cite web | |||
| Unlike laypeople, professionals in virtually all engineering and scientific disciplines involving accelerations and ] rigorously maintain the distinctions between mass, force, and weight, as well as their respective units of measure. Engineers in disciplines involving ] ] (force on a structure due to gravity), such as ], first convert loads due to objects like concrete and automobiles—which are always tallied in kilograms—to newtons before continuing with their calculations. Primarily, this is because material properties like ] are quite properly measured and published in terms of newtons and ]s (which is a unit of pressure derived from the newton). Mass in kilograms is converted to weight in newtons by multiplying by gravitational acceleration (see '']'', above). | |||
| |url = http://www.bipm.org/utils/en/pdf/si_brochure_draft_ch2.pdf | |||
| |title = Draft Chapter 2 for SI Brochure, following redefinitions of the base units | |||
| |author = Ian Mills | |||
| |publisher = CCU | |||
| |date = September 29, 2010 | |||
| |access-date =January 1, 2011 | |||
| }}</ref> This resolution was accepted by the 24th conference of the CGPM<ref> | |||
| {{cite conference | |||
| |url= http://www.bipm.org/utils/en/pdf/24_CGPM_Resolution_1.pdf | |||
| |title= Resolution 1 – On the possible future revision of the International System of Units, the SI | |||
| |conference= 24th meeting of the General Conference on Weights and Measures | |||
| |location = Sèvres, France | |||
| |date = October 17–21, 2011 | |||
| |access-date =October 25, 2011 | |||
| }}</ref> in October 2011 and further discussed at the 25th conference in 2014.<ref name=":0">{{Cite web|url=http://www.bipm.org/en/CGPM/db/25/1/|title=BIPM – Resolution 1 of the 25th CGPM|website=www.bipm.org|access-date=March 27, 2017}}</ref><ref> | |||
| {{cite press release | |||
| | url = http://www.bipm.org/utils/en/pdf/Press_release_resolution_1_CGPM.pdf | |||
| | title = General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram. | |||
| | publisher = ] | |||
| | location = Sèvres, France | |||
| | date = October 23, 2011 | |||
| | access-date = October 25, 2011 | |||
| }}</ref> Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018.<ref name=":0" /> Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The ] is one way to do this.<ref>{{cite journal |doi=10.1088/0026-1394/53/5/A46 |title=The watt or Kibble balance: A technique for implementing the new SI definition of the unit of mass |journal=Metrologia |volume=53 |issue=5 |pages=A46–A74 |year=2016 |last1=Robinson |first1=Ian A. |last2=Schlamminger |first2=Stephan |pmid=35023879 |pmc=8752041 |bibcode=2016Metro..53A..46R |doi-access=free }}</ref> | |||
| As part of this project, a variety of very ] were considered and explored over many years. Some of these approaches were based on equipment and procedures that would enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and that expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. All approaches depend on converting a weight measurement to a mass and therefore require precise measurement of the strength of gravity in laboratories (]). All approaches would have precisely fixed one or more constants of nature at a defined value.{{Citation needed|date=July 2023}} | |||
| ====Buoyancy and “conventional mass”==== | |||
| ]<!-- | |||
| == SI multiples == | |||
| -->The masses of objects are relatively invariant whereas their weights vary slightly with changes in barometric pressure, such as with changes in weather and altitude. This is because objects have ] and therefore have a ] effect in air. Buoyancy—a force that counters gravity’s—reduces the weight of all objects immersed in ]s. Further, objects with precisely the same mass but with different ] displace different volumes and therefore have different buoyancies and weights. Normally, the effect of air buoyancy is too small to be of any consequence in normal day-to-day activities.<ref>For instance, buoyancy’s diminishing effect upon one’s body weight (a relatively low-density object) is 1/860<sup>th</sup> that of gravity. Variations in barometric pressure rarely affect one’s weight more than ±1 part in 30,000. Assumptions: An air density of 1160 g/m³, an average density of a human body (with collapsed lungs) equal to that of water, and variations in barometric pressure rarely exceeding ±22 torr. Assumptions primary variables: An altitude of 194 meters above mean sea level (the worldwide median altitude of human habitation), an indoor temperature of 23 °C, a dewpoint of 9 °C, and 760 mmHg sea level–corrected barometric pressure.</ref> In ] however, where mass standards are calibrated with extreme accuracy, buoyancy is a significant effect so air density is accurately accounted for during calibration. | |||
| {{main|Orders of magnitude (mass)}}<!--NOTE TO EDITORS: This section is linked from ] as well as many other Misplaced Pages articles. Please do not delete or rename without fixing them.--> | |||
| {{Redirect|Milligram|the American band|Milligram (band)|the Serbian band|Miligram (band)|the horse|Milligram (horse)}} | |||
| Because an SI unit may not have multiple prefixes (see ]), prefixes are added to '']'', rather than the base unit ''kilogram'', which already has a prefix as part of its name.<ref>BIPM: SI Brochure: Section 3.2, '''' {{Webarchive|url=https://web.archive.org/web/20160329162832/http://www.bipm.org/en/publications/si-brochure/section3-2.html |date=March 29, 2016 }}</ref> For instance, one-millionth of a kilogram is 1{{nbsp}}mg (one milligram), not 1{{nbsp}}μkg (one microkilogram). | |||
| <div style="float:center; margin-left: 1em;"> | |||
| {{SI multiples | |||
| | unit = gram | |||
| | symbol = g | |||
| | p= | n= | mc= | m= | k= | M= | G= | T= | |||
| | note = Common prefixed units are in bold face.<ref group="Note">Criterion: A combined total of at least five occurrences on the ] and the ], including both the singular and the plural for both the -''gram'' and the -''gramme'' spelling.</ref> | |||
| }} | |||
| </div> | |||
| == Practical issues with SI weight names == | |||
| Given the extremely high cost of ]-] prototypes, high-quality “working” standards are made of special ] alloys, which occupy greater volume than those made of platinum-iridium. For convenience, a standard value of buoyancy relative to stainless steel was developed for metrology work and this results in the term “conventional mass.”<Ref>''International Recommendation OIML R33''. See Web site.</ref> Conventional mass is defined as follows: “For a mass at 20 °C, ‘conventional mass’ is the mass of a reference standard of density 8000 kg/m³ which it balances in air with a density of 1.2 kg/m³.” The effect is a small one, 150 ] for stainless steel mass standards, but the appropriate corrections are made during the calibration of all precision mass standards so they have the true mass indicated on them. In routine laboratory use however, the reading on a precision scale when a stainless steel standard is placed upon it is actually its conventional mass; that is, its true mass minus buoyancy. Also, any object compared to a stainless steel mass standard has ''its'' conventional mass measured; that is, its true mass minus some (usually unknown) degree of buoyancy. | |||
| <!--EDITORS NOTE REGARDING BULLET-POINT TEXT SIZE: The below bullet notes have alternately been tried in both normal-size text, then small text, then normal, etc. While it may be tempting to reduce the below section (as they are near-parenthetical, footnote-type notes), small text has proven too difficult to read on many computer systems. Accordingly, please leave the below text in normal size. --> | |||
| * Serious medication errors have been made by confusing milligrams and micrograms when micrograms has been abbreviated.<ref name="SPCG">{{cite web |url=http://www.palliativecareguidelines.scot.nhs.uk/guidelines/about-the-guidelines/pharmacological-considerations/prescribing-information-for-liquid-medicines.aspx |title=Prescribing Information for Liquid Medicines |website=Scottish Palliative Care Guidelines |access-date=June 15, 2015 |archive-url=https://web.archive.org/web/20180710152658/http://www.palliativecareguidelines.scot.nhs.uk/guidelines/about-the-guidelines/pharmacological-considerations/prescribing-information-for-liquid-medicines.aspx |archive-date=July 10, 2018 |url-status=dead }}</ref> The abbreviation "mcg" rather than the SI symbol "μg" is formally mandated for medical practitioners in the US by the ] (JCAHO).<ref>{{cite web |title=New Joint Commission "Do Not Use" List: Abbreviations, Acronyms, and Symbols |url=http://www.aapmr.org/practice/guidelines/Pages/New-Joint-Commission-symbols.aspx |publisher=American Academy of Physical Medicine and Rehabilitation |access-date=19 February 2024 |archive-url=https://web.archive.org/web/20150915012112/http://www.aapmr.org/practice/guidelines/Pages/New-Joint-Commission-symbols.aspx |archive-date=15 September 2015}}</ref> In the United Kingdom, the ] and Scottish Palliative Care Guidelines state that "micrograms" and "nanograms" must both be written in full, and never abbreviated as "mcg" or "μg".<ref name="SPCG" /><ref>{{cite web |title=Prescription writing |url=https://bnf.nice.org.uk/medicines-guidance/prescription-writing/ |publisher=] |access-date=19 February 2024}}</ref> | |||
| * The hectogram (100 g) (Italian: ''ettogrammo'' or ''etto'') is a very commonly used unit in the retail food trade in Italy.<ref>Tom Stobart, ''The Cook's Encyclopedia'', 1981, p. 525</ref><ref>J.J. Kinder, V.M. Savini, ''Using Italian: A Guide to Contemporary Usage'', 2004, {{isbn|0521485568}}, p. 231</ref><ref>Giacomo Devoto, Gian Carlo Oli, ''Nuovo vocabolario illustrato della lingua italiana'', 1987, ''s.v.'' 'ètto': "frequentissima nell'uso comune: ''un e. di caffè, un e. di mortadella; formaggio a 2000 lire l'etto''"</ref> | |||
| * The former standard spelling and abbreviation "deka-" and "dk" produced abbreviations such as "dkm" (dekametre) and "dkg" (dekagram).<ref>U.S. National Bureau of Standards, ''The International Metric System of Weights and Measures'', "Official Abbreviations of International Metric Units", 1932, </ref> {{As of|2020|post=,}} the abbreviation "dkg" (10 g) is still used in parts of central Europe in retail for some foods such as cheese and meat.<ref name="Czeck dkg ham">{{cite web |title=Jestřebická hovězí šunka 10 dkg {{!}} Rancherské speciality |url=https://eshop.rancherskespeciality.cz/Jestrebicka-hovezi-sunka-10-dkg-d189.htm |website=eshop.rancherskespeciality.cz |language=cs|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616032253/https://eshop.rancherskespeciality.cz/Jestrebicka-hovezi-sunka-10-dkg-d189.htm|archive-date=June 16, 2020}}</ref><ref name="Slovak dkg ham">{{cite web |title=Sedliacka šunka 1 dkg {{!}} Gazdovský dvor – Farma Busov Gaboltov |url=http://farmabusoveshop.sk/sedliacka-sunka-1-dkg |website=Sedliacka šunka 1 dkg |language=sk|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616033111/http://farmabusoveshop.sk/sedliacka-sunka-1-dkg|archive-date=June 16, 2020}}</ref><ref name="Czech dkg cheese">{{cite web |title=sýr bazalkový – Farmářské Trhy |url=http://www.e-farmarsketrhy.cz/syry-z-kravskeho-mleka/syr-bazalkovy |website=www.e-farmarsketrhy.cz |language=cs|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616033522/http://www.e-farmarsketrhy.cz/syry-z-kravskeho-mleka/syr-bazalkovy|archive-date=June 16, 2020}}</ref><ref name="Hungarian menu">{{cite web |title=English Menu – Cafe Mediterran |url=http://www.mediterrangrill.hu/english-menu/ |language=en|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616034445/http://www.mediterrangrill.hu/english-menu/|archive-date=June 16, 2020|quote= Beef steak 20 dkg; Beef steak 40 dkg;Thick crust 35 dkg}}</ref><ref name="Hungarian dkg cheese">{{cite web |title=Termékek – Csíz Sajtműhely |url=https://csizsajtmuhely.hu/sajtrendeles/ |language=hu|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616035724/https://csizsajtmuhely.hu/sajtrendeles/|archive-date=June 16, 2020}}</ref> | |||
| * The unit name ''megagram'' is rarely used, and even then typically only in technical fields in contexts where especially rigorous consistency with the SI standard is desired. For most purposes, the name '']'' is instead used. The tonne and its symbol, "t", were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. According to the BIPM, "This unit is sometimes referred to as 'metric ton' in some English-speaking countries."<ref>''Non-SI units that are accepted for use with the SI'', , BIPM</ref> | |||
| == See also == | |||
| The effect of buoyancy invalidates the standard answer to the childhood riddle of “Which weighs more, a ton of lead or a ton of feathers (or aluminum)?” The standard answer is that they both weigh the same, but the correct answer is “Lead weighs more than aluminum, by 327 grams-force or 3.21 newtons (a difference of 0.0327%).” This is because the density of lead is greater and displaces less air.<ref>If true mass in metric tons is measured. If ''conventional mass'' is used (no compensations for buoyancy are made), their weights will be identical. Value assumes standard gravity and an air density of 1160 g/m³.</ref> | |||
| {{Portal|Physics}} | |||
| * {{Annotated link|Inertia}} | |||
| * {{Annotated link|Kibble balance}} | |||
| * {{Annotated link|Kilogram-force}} | |||
| * {{Annotated link|Mass versus weight}} | |||
| * {{Annotated link|Metric system}} | |||
| * {{Annotated link|National Institute of Standards and Technology}} (NIST) | |||
| * {{Annotated link|Newton (unit)|Newton}} | |||
| * {{Annotated link|Standard gravity}} | |||
| * {{Annotated link|Weight}} | |||
| == Notes == | |||
| ====Types of scales and what they measure==== | |||
| {{reflist|group="Note"}} | |||
| It’s notable at a purely technical level, that whenever someone stands on a balance-beam scale at a doctor’s office, they are really and truly having their mass measured. Excluding buoyancy, which affects all types of scales in ]s, balance-beam scales compare the mass on the platform with those of the sliding counterweights on the beams; gravity serves only as the force-generating mechanism that allows the needle to diverge from the “balanced” (null) point. On scales such as these, gravity can vary in strength without affecting the reading. Conversely, whenever someone steps onto a spring-based or digital ]-based scale, they are technically having their ''weight'' measured notwithstanding that the displayed units of measure are in kilograms. On force-measuring instruments such as these, variations in gravity will affect the reading. As a practical matter, when force-measuring scales are used in commerce or hospitals, they are calibrated on-site and certified on that basis.<ref>National General Conference on Weights and Measures, ''Specifications, Tolerances, and Other Technical Requirements for Weighing and Measuring Devices,'' NIST Handbook 44</ref> | |||
| == References == | |||
| == Proposed future definitions == | |||
| {{reflist|30em}} | |||
| <!--NOTE TO EDITORS: This section is externally linked to from other places in the article. Please do not delete or rename without fixing the referencing links.--> | |||
| == External links == | |||
| :''In the following section, wherever numeric equalities are shown in ‘concise form’ — such as'' 1.854<font size="-1"> </font>87(14) × 10<sup>43</sup>'' — the two digits between the parentheses denotes the ] (the ] at ''68.27%'' confidence level) in the two least significant digits of the mantissa.'' | |||
| {{Commons category}} | |||
| {{External media | |||
| The kilogram is the only SI unit that is still defined in relation to an artifact. Note that the ] was also once defined as an artifact (a single platinum-iridium bar with two marks on it). However, it was eventually redefined in terms of invariant, fundamental constants of nature that are delineated via ''practical realizations'' (apparatus) that can be reproduced in different laboratories by following a written specification. Today, physicists are investigating various approaches to do the same with the kilogram. Some of the approaches are fundamentally very different from each other. Some are based upon equipment and procedures that enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, fundamental constants. Others are essentially devices that measure either the acceleration or weight of hand-tuned, kilogram test masses and which express their ] in electrical terms via special components that permit traceability to fundamental constants. Measuring the weight of test masses requires the precise measurement of the strength of gravity in laboratories. All approaches but one, the ''Avogadro Project'', would precisely fix one or more constants of nature at a defined value. These different approaches are as follows: | |||
| | float = right | |||
| | width = 40% | |||
| ====Atom-counting approaches==== | |||
| | image2 = NIST: resting on an egg crate fluorescent light panel | |||
| =====Avogadro project===== | |||
| | image3 = BIPM: '''' | |||
| ].]]<!-- | |||
| | image4 = BIPM: | |||
| | image5 = The Age: | |||
| -->An ]-based approach, known as the ''Avogadro project'', attempts to define and delineate the kilogram as a quantity of ] atoms. Silicon was chosen because a commercial infrastructure with mature processes for creating defect-free, ultra-pure monocrystalline silicon already exists to service the ] industry. To make a practical realization of the kilogram, a silicon ] (a rod-like, single-crystal ingot) would be produced. Its ] composition would be measured with a ] to determine its average atomic mass. The rod would be cut, ground, and polished into spheres. The size of a select sphere would be measured using optical ]. The precise lattice spacing between the atoms in its crystal structure (≈192 pm) would be measured using a scanning X-ray interferometer. Amazingly, this permits its atomic spacing to be determined with an uncertainty of only three parts in a billion. With the size of the sphere, its average atomic mass, and the atomic spacing known, the required number of atoms in the sphere could be calculated with sufficient precision and uncertainty to enable it to be ground down to the desired quantity of atoms (mass). | |||
| | image6 = NPL: | |||
| | image7 = NIST: This particular , an Austrian-made precision balance, was used by the NIST from 1945 until 1960 | |||
| Experiments are planned for the Avogadro Project’s silicon sphere to determine whether its mass is most stable when stored in a vacuum, a partial vacuum, or ambient pressure. However, no technical means currently exist to prove a stability any better than that of the IPK’s because the most sensitive and accurate measurements of mass are made with balances like the BIPM’s FB-2 flexure-strip balance (see '']'', below). Balances can only compare the mass of a silicon sphere to that of a reference mass. Given the latest understanding of the lack of relative stability between the IPK and its replicas, there is no known, perfectly stable mass artifact to compare against. Scales capable of measuring mass relative to an invariant of nature at the 30-parts-per-billion level of uncertainty of the IPK do not yet exist. Another issue to be overcome is that silicon oxidizes and forms a thin layer of ] (common glass). This layer slightly increases the mass of the sphere and its effect must be accounted for when grinding the sphere to its finish dimension. Oxidation is not an issue with platinum and iridium, both of which are noble metals that are roughly as ] as oxygen and therefore don’t oxidize unless coaxed to do so in the laboratory. The presence of a thin glass layer on a silicon-sphere mass prototype places additional restrictions on the procedures that might be suitable to clean it. | |||
| | image8 = BIPM: , the BIPM's modern precision balance featuring a standard deviation of one ten-billionth of a kilogram (0.1{{nbsp}}μg) | |||
| | image9 = BIPM: , featuring 1{{nbsp}}μg resolution and a 4{{nbsp}}kg maximum mass. Also used by NIST and Sandia National Laboratories' Primary Standards Laboratory | |||
| =====Ion accumulation===== | |||
| | image10 = Micro-g LaCoste: , (), used in national laboratories to measure gravity to 2{{nbsp}}]<!--NOTE TO EDITORS: The Micro-g LaCoste Web site does not display properly on some computer systems. The accuracy is NOT 2 mGal and is indeed 2 μGal. Please see discussion topic at ]--> accuracy | |||
| Another Avogadro-based approach, ] accumulation, would define and delineate the kilogram by creating metal mass artifacts. It would do so by accumulating of ] or ] ]s (atoms stripped of an electron) and counting them by measuring the electrical current required to neutralize the ions. Gold and bismuth are used because, unlike most other elements, they each have only one naturally occurring ]. | |||
| }} | |||
| * | |||
| With a gold-based definition of the kilogram for instance, the definition of the ] would be changed from one based on a ] to the quantity of atoms as are in 196.966<font size="-1"> </font>569 g of gold (from the current value of 196.966<font size="-1"> </font>569(4) grams) and the kilogram would be defined as “the mass equal to that of precisely 1000/196.966569 moles (≅5.077<font size="-1"> </font>003<font size="-1"> </font>7021 moles) of gold atoms.” | |||
| * The UK's National Physical Laboratory (NPL): | |||
| * NPL: '''' | |||
| Ion-accumulation techniques, while a relatively new field of study, have advanced rapidly. In 2003, experiments with gold at a current of only 10 µa demonstrated a relative uncertainty of 1.5%. Yet, follow-on experiments using bismuth ions and a current of 30 mA were expected to accumulate a mass of 30 g in six days and to have a relative uncertainty of better than 1 part in 10<sup>6</sup>.<ref>''General Conference on Weights and Measures, 22nd Meeting, October 2003'' ().</ref> | |||
| * Metrology in France: '' {{Webarchive|url=https://web.archive.org/web/20140319084923/http://www.french-metrology.com/en/feature/watt-balance.asp |date=March 19, 2014 }}'' | |||
| * Australian National Measurement Institute: '''' | |||
| The difficulty with ion-accumulation-based standards is in obtaining truly practical mass artifacts. Gold, while dense and a ] (resistant to oxidation and the formation of other compounds), is extremely soft so mass artifacts would require extraordinary care to avoid wear. Bismuth, while an inexpensive metal for experiments, would not produce stable artifacts because it readily oxidizes and forms other chemical compounds. Iridium and platinum are composed of two and six isotopes respectively and this places an upper limit on relative uncertainty. | |||
| * International Bureau of Weights and Measures (BIPM): | |||
| =====Carbon-12===== | |||
| Though not offering a practical realization, this definition would precisely define the magnitude of the kilogram in terms of a certain number of ] atoms. The ] is currently defined as “the quantity of ‘entities’ (elementary particles like atoms or molecules) as there are atoms in 12 grams of carbon-12.” Thus, the current definition of the mole requires that 1000/12 (83⅓) moles of C-12 has a mass of precisely one kilogram. The number of atoms in a mole, a quantity known as the ], is an experimentally determined value that is currently measured as being 6.022<font size="-1"> </font>141<font size="-1"> </font>79(30) × 10<sup>23</sup> atoms (2006 CODATA value). This new definition of the kilogram proposes to fix the Avogadro constant at precisely 6.022<font size="-1"> </font>141<font size="-1"> </font>79 × 10<sup>23</sup> and the kilogram would be defined as “the mass equal to that of 83⅓ × 6.022<font size="-1"> </font>141<font size="-1"> </font>79 × 10<sup>23</sup> atoms of carbon-12.” | |||
| Currently, the uncertainty in the Avogadro constant is determined by the uncertainty in the measured mass of carbon-12 atoms (a relative standard uncertainty of 50 parts per billion at this time). By fixing the Avogadro constant, the practical effect of this proposal would be that the precise magnitude of the kilogram would be subject to future refinement as improved measurements of the mass of carbon-12 atoms become available. Electronic realizations of the kilogram would be recalibrated as required. In an electronic ''definition'' of the kilogram, the mole would remain decoupled from the kilogram and could continue to be defined in terms of 12 grams of carbon-12. In that case, 83⅓ moles of carbon-12 would—by definition—continue to have a mass of precisely one kilogram and the Avogadro constant would continue to have uncertainty in its precise value. | |||
| A variation on a carbon-12-based definition proposes to define the Avogadro constant as being precisely 84,446,886<sup>3</sup> | |||
| (≅6.022<font size="-1"> </font>140<font size="-1"> </font>98 × 10<sup>23</sup>) atoms. An imaginary realization of a 12-gram mass prototype would be a cube of carbon-12 atoms measuring precisely 84,446,886 atoms across on a side. With this proposal, the kilogram would be defined as “the mass equal to 84,446,886<sup>3</sup> × 83⅓ atoms of carbon-12.” The value 84,446,886 was chosen because it has a special property; its cube (the proposed new value for the Avogadro constant) is evenly divisible by twelve. Thus with this definition of the kilogram, there would be an integer number of atoms in one gram of carbon-12: 50,184,508,190,229,061,679,538 atoms.<!-- | |||
| --><ref>Georgia Tech, 21 Sept. 2007 press release: '''' Note that the uncertainty in the Avogadro constant narrowed since this proposal was first submitted to '']'' for publication. The 2006 CODATA value for the Avogadro constant has a relative standard uncertainty of 50 parts per billion and the only cube root values within this uncertainty must fall within the range of 84,446,889.8 ±1.4; that is, there are only three integer cube roots (…89, …90, and …91) in this range and the value 84,446,886 falls outside of it. Unfortunately, none of the three integer values within the range posses the property of their cubes being divisible by twelve; one gram of C-12 could not comprise an integer number of atoms. If the value 84,446,886 was adopted to define the kilogram, many other constants of nature and electrical units would have to be revised an average of about 0.13 part per million. The straightforward adjustment to this approach advanced by the group would instead define the kilogram as “the mass equal to 84,446,890<sup>3</sup> × 83⅓ atoms of carbon-12.” This proposed value for the Avogadro constant falls neatly within the measured value (≅6.022<font size="-1"> </font>141<font size="-1"> </font>84 × 10<sup>23</sup> vs. the 2006 CODATA value of 6.022<font size="-1"> </font>141<font size="-1"> </font>79(30) × 10<sup>23</sup>) and the proposed definition of the kilogram produces an integer number of atoms in 12 grams of carbon-12, but not for 1 gram nor 1 kilogram.</ref> | |||
| ====Electronic approaches==== | |||
| =====Watt balance===== | |||
| ] watt balance is a project of the U.S. Government to develop an “electronic kilogram.” The vacuum chamber dome, which lowers over the entire apparatus, is visible at top.]]The ] is essentially an ] with an extra calibration step that nulls the effect of geometry. The electrical current in the watt balance is delineated by a ], which allows voltages to be linked to an invariant constant of nature with extremely high precision and stability, and the circuit resistance is calibrated against a ]. The watt balance requires ''exquisitely precise'' measurement of gravity in a laboratory (see “FG-5 absolute gravimeter” in '']'', below) and compares this acceleration to the electrical power necessary to counter it. For instance, the gravity gradient of 3.1 ]/cm (3 parts in 10<sup>9</sup>) is accounted for when the elevation of the center of the ] differs from that of the nearby test mass. As of late 2007, the ] implementation of the watt balance was approaching the level where scientists could resolve a difference of about 25 µg. Ultimately, the watt balance would define the kilogram in terms of the ], which is a measure that relates the energy of photons to their frequency. | |||
| In the watt balance, the Planck constant would be fixed, where ''h'' = 6.626<font size="-1"> </font>068<font size="-1"> </font>96 × 10<sup>–34</sup> ]·] (from the 2006 ] value of 6.626<font size="-1"> </font>068<font size="-1"> </font>96(33) × 10<sup>–34</sup> J·s) and the kilogram would be defined as “the mass of a body at rest whose equivalent energy equals the energy of photons whose frequencies sum to 1.356<font size="-1"> </font>392<font size="-1"> </font>733 × 10<sup>50</sup> Hz.”<ref>''Hysteresis and Related Error Mechanisms in the NIST Watt Balance Experiment'', Joshua P. Schwarz ''et al.'', Journal of Research of the National Bureau of Standards and Technology, '''106''', No. 4, July–August 2001 (); and ''On the redefinition of the kilogram'', B. N. Taylor ''et al.'', Metrologia '''36''' (1999), 63–64; and the .</ref> | |||
| The virtue of electronic-based realizations like the watt balance is that the definition of the kilogram would not be based on carefully stored and handled kilogram prototypes. It would free physicists from the need to rely on assumptions about the stability of those prototypes, including those that would be manufactured under atom-counting schemes. Instead, hand-tuned, close-approximation mass standards would simply be weighed and documented as being equal to one kilogram plus an offset value. With scales, the kilogram would not only be ''defined'' in electrical terms, it would also be ''delineated'' in electrical terms. Mass artifacts calibrated in a watt balance would effectively become '']''. Further, one additional term in all scale-based realizations — acceleration due to gravity — is currently measured using dropping-mass absolute gravimeters that contain an iodine-stabilized ] ]. The fringe-signal, frequency-sweep output from the interferometer is measured with a rubidium ]. Thus, the “gravity” term in the delineation of an all-electronic kilogram would also be measured relative to invariants of nature. | |||
| Scales also permit more flexibility in choosing materials with especially desirable properties for mass standards. For instance, 90Pt–Ir could continue to be used so that the specific gravity of newly produced mass standards would be the same as existing national primary and check standards. This would reduce the relative uncertainty when making mass comparisons in air. Alternately, entirely different materials and constructions could be explored with the objective of producing mass standards with greater stability. For instance, ]-iridium alloys could be investigated if platinum’s propensity to absorb ] and ] (due to ] of ] and ]-based cleaning solvents) proved to be sources of instability. Also, vapor-deposited, protective ceramic coatings like ]s could be investigated for their suitability to isolate these new alloys. | |||
| =====Ampere-based force===== | |||
| This approach would define the kilogram as “the mass which would be accelerated at precisely 2 × 10<sup>–7</sup> m/s² when subjected to the per-meter force between two straight parallel conductors of infinite length, of negligible circular cross section, placed 1 meter apart in vacuum, through which flow a constant current of <sup><font size="-1">1</font></sup>/<sub><font size="-1">1.602</font><font size="-2"> </font><font size="-1">176</font><font size="-2"> </font><font size="-1">487 × 10</font></sup><font size="-2">–19</font> (6,241,509,647,120,417,390) elementary charges per second.” | |||
| Effectively, this would define the kilogram as a derivative of the ], rather than present relationship, which defines the ampere as a derivative of the kilogram. This redefinition of the kilogram would result from fixing the ] (''e'') to be precisely 1.602<font size="-1"> </font>176<font size="-1"> </font>487 × 10<sup>–19</sup> ] (from the current 2006 CODATA value of 1.602<font size="-1"> </font>176<font size="-1"> </font>487(40) × 10<sup>–19</sup>), which effectively defines the coulomb as being the sum of 6,241,509,647,120,417,390 elementary charges. It would necessarily follow that the ampere then becomes an electrical current of this same quantity of elementary charges per second. | |||
| The virtue of a practical realization based upon this definition is that unlike the watt balance and other scale-based methods, all of which require the careful characterization of gravity in the laboratory, this method delineates the magnitude of the kilogram directly in the very terms that define the nature of mass: acceleration due to an applied force. Unfortunately, it is extremely difficult to develop a practical realization based upon accelerating masses. Experiments over a period of years in Japan with a ], 30-gram mass supported by diamagnetic levitation never achieved an uncertainty better than 10 parts in 10<sup>6</sup>. ] was one of the limiting issues. Other groups are continuing this line of research using different techniques to levitate the mass.<ref>NIST, ''''; and ''A Watt Balance On Its Side'', R. Steiner, NIST, 24 Sept. 2007.</ref> | |||
| == See also == | |||
| {{col-begin}} | |||
| {{col-break}} | |||
| * ] | |||
| * ] (SI) | |||
| * ] (BIPM) | |||
| * ] (CIPM) | |||
| * ] | |||
| * ] (CGPM) | |||
| * ] | |||
| * ] (orig. name of the kilogram, history of) | |||
| * ] | |||
| * ] | |||
| {{col-break}} | |||
| * ] | |||
| * ] | |||
| * ] (NIST) | |||
| * ] | |||
| * ]s | |||
| * ]s | |||
| * ] | |||
| * ] (metric ton) | |||
| * ] | |||
| * ] | |||
| {{col-end}} | |||
| ==Notes== | |||
| <references /> | |||
| ==External links== | |||
| * NIST: '''' | |||
| * The U.K.’s National Physical Laboratory (NPL): | |||
| * NPL: '''' | |||
| * Australian National Measurement Institute: '''' | |||
| * BIPM home page: | |||
| * NZZ Folio: '''' | * NZZ Folio: '''' | ||
| * NPL: '''' | * NPL: '''' | ||
| * BBC: '''' | |||
| * NPR: '''', an interview with ] physicist Richard Steiner | |||
| * | |||
| * | |||
| * {{cite news |url=https://www.theguardian.com/science/2018/nov/09/in-the-balance-scientists-vote-on-first-change-to-kilogram-in-century |title=In the balance: scientists vote on first change to kilogram in a century |newspaper=] |first=Ian |last=Sample |date=November 9, 2018 |access-date=November 9, 2018}} | |||
| === |
=== Videos === | ||
| * – ] channel | |||
| * BIPM: | |||
| * on ] | |||
| * NIST: , resting on an egg crate fluorescent light panel | |||
| * BIPM: '''' | |||
| * BIPM: | |||
| * The Age: | |||
| * NIST: , Austrian-made, precision balance used by the NIST from 1945 until 1960 | |||
| * BIPM: , the BIPM’s modern precision balance featuring a standard deviation of one ten-billionth of a kilogram (0.1 µg) | |||
| * BIPM: , featuring 1 µg resolution. Also used by NIST and Sandia National Laboratories’ Primary Standards Laboratory | |||
| * Micro-g LaCoste: (), used in national labs to measure gravity to 2 ]<!--NOTE TO EDITORS: The Micro-g LaCoste Web site does not display properly on some computer systems. The accuracy is NOT 2 mGal and is indeed 2 µGal. Please see discussion topic at ]--> accuracy | |||
| {{SI units}} | |||
| ==Glossary==<!--NOTE TO EDITORS: This section is not intended to be all-inclusive. It comprises only those words and terms with very specific and/or unique meanings in the discipline of mass metrology. | |||
| {{Authority control}} | |||
| --> | |||
| {{Good article}} | |||
| * '''Artifact''': A human-made object used as a comparative standard in the measurement of a physical quantity. | |||
| * '''Check standard''': | |||
| :# A standard body’s backup replica of the IPK. | |||
| :# A secondary kilogram mass standard used for routine calibrations. | |||
| * '''Definition''': A formal, specific, and exact specification. | |||
| * '''Delineate''': To use physical means to mark a boundary or express the magnitude of an entity. | |||
| * '''Delineation''': The physical means used to mark a boundary or express the magnitude of an entity. | |||
| * '''Magnitude''': The extent or numeric value of a property | |||
| * '''National prototype''': A replica of the IPK possessed by a nation. | |||
| * '''Practical realization''': An artifact or readily reproducible apparatus for delineating the magnitude of a unit of measure. | |||
| * '''Primary national standard''': | |||
| :# A replica of the IPK possessed by a nation | |||
| :# The least used replica of the IPK when a nation possesses more than one. | |||
| * '''Prototype''': | |||
| :# A human-made object that serves as the defining comparative standard in the measurement of a physical quantity. | |||
| :# A human-made object that serves as ''the'' comparative standard in the measurement of a physical quantity. | |||
| :# The IPK and any of its replicas | |||
| * '''Replica''': An official copy of the IPK. | |||
| * '''Transfer standard''': An artifact or apparatus that reproduces the magnitude of a unit of measure in a different, usually more practical, form. | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:02, 6 January 2025
Metric unit of mass "kg" redirects here. For other uses, see KG.
| kilogram | |
|---|---|
 A series of 5, 2, 1, 0.5 and 0.2 kilogram weights, made of cast iron A series of 5, 2, 1, 0.5 and 0.2 kilogram weights, made of cast iron | |
| General information | |
| Unit system | SI |
| Unit of | mass |
| Symbol | kg |
| Conversions | |
| 1 kg in ... | ... is equal to ... |
| Avoirdupois | |
| British Gravitational | ≈ 0.0685 slugs |
| CGS units | 1000 g |
| Daltons | 6.02214076×10 Da |
The kilogram (also spelled kilogramme) is the base unit of mass in the International System of Units (SI), having the unit symbol kg. 'Kilogram' means 'one thousand grams' and is colloquially abbreviated to kilo.
The kilogram is an SI base unit, defined ultimately in terms of three defining constants of the SI, namely a specific transition frequency of the caesium-133 atom, the speed of light, and the Planck constant. A properly equipped metrology laboratory can calibrate a mass measurement instrument such as a Kibble balance as a primary standard for the kilogram mass.
The kilogram was originally defined in 1795 during the French Revolution as the mass of one litre of water. The current definition of a kilogram agrees with this original definition to within 30 parts per million. In 1799, the platinum Kilogramme des Archives replaced it as the standard of mass. In 1889, a cylinder composed of platinum–iridium, the International Prototype of the Kilogram (IPK), became the standard of the unit of mass for the metric system and remained so for 130 years, before the current standard was adopted in 2019.
Definition
The kilogram is defined in terms of three defining constants:
- a specific atomic transition frequency ΔνCs, which defines the duration of the second,
- the speed of light c, which when combined with the second, defines the length of the metre,
- and the Planck constant h, which when combined with the metre and second, defines the mass of the kilogram.
The formal definition according to the General Conference on Weights and Measures (CGPM) is:
The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.62607015×10 when expressed in the unit J⋅s, which is equal to kg⋅m⋅s, where the metre and the second are defined in terms of c and ΔνCs.
— CGPM
Defined in term of those units, the kg is formulated as:
kg = (299792458)/(6.62607015×10)(9192631770)hΔνCs/c kg ≈ (1.475521399735270×10)hΔνCs/c.This definition is generally consistent with previous definitions: the mass remains within 30 ppm of the mass of one litre of water.
Timeline of previous definitions

- 1793: The grave (the precursor of the kilogram) was defined as the mass of 1 litre (dm) of water, which was determined to be 18841 grains.
- 1795: the gram (/1000 of a kilogram) was provisionally defined as the mass of one cubic centimetre of water at the melting point of ice.
- 1799: The Kilogramme des Archives was manufactured as a prototype. It had a mass equal to the mass of 1 dm of water at the temperature of its maximum density, which is approximately 4 °C.
- 1875–1889: The Metre Convention was signed in 1875, leading to the production of the International Prototype of the Kilogram (IPK) in 1879 and its adoption in 1889.
- 2019: The kilogram was defined in terms of the Planck constant, the speed of light and hyperfine transition frequency of Cs as approved by the General Conference on Weights and Measures (CGPM) on 16 November 2018.
Name and terminology
The kilogram is the only base SI unit with an SI prefix (kilo) as part of its name. The word kilogramme or kilogram is derived from the French kilogramme, which itself was a learned coinage, prefixing the Greek stem of χίλιοι khilioi "a thousand" to gramma, a Late Latin term for "a small weight", itself from Greek γράμμα. The word kilogramme was written into French law in 1795, in the Decree of 18 Germinal, which revised the provisional system of units introduced by the French National Convention two years earlier, where the gravet had been defined as weight (poids) of a cubic centimetre of water, equal to 1/1000 of a grave. In the decree of 1795, the term gramme thus replaced gravet, and kilogramme replaced grave.
The French spelling was adopted in Great Britain when the word was used for the first time in English in 1795, with the spelling kilogram being adopted in the United States. In the United Kingdom both spellings are used, with "kilogram" having become by far the more common. UK law regulating the units to be used when trading by weight or measure does not prevent the use of either spelling.
In the 19th century the French word kilo, a shortening of kilogramme, was imported into the English language where it has been used to mean both kilogram and kilometre. While kilo as an alternative is acceptable, to The Economist for example, the Canadian government's Termium Plus system states that "SI (International System of Units) usage, followed in scientific and technical writing" does not allow its usage and it is described as "a common informal name" on Russ Rowlett's Dictionary of Units of Measurement. When the United States Congress gave the metric system legal status in 1866, it permitted the use of the word kilo as an alternative to the word kilogram, but in 1990 revoked the status of the word kilo.
The SI system was introduced in 1960 and in 1970 the BIPM started publishing the SI Brochure, which contains all relevant decisions and recommendations by the CGPM concerning units. The SI Brochure states that "It is not permissible to use abbreviations for unit symbols or unit names ...".
For use with east Asian character sets, the SI symbol is encoded as a single Unicode character, U+338F ㎏ SQUARE KG in the CJK Compatibility block.
Redefinition based on fundamental constants
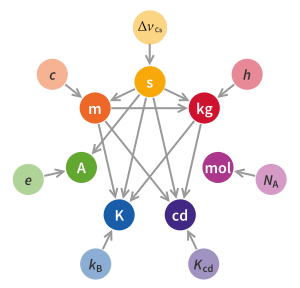

The replacement of the International Prototype of the Kilogram (IPK) as the primary standard was motivated by evidence accumulated over a long period of time that the mass of the IPK and its replicas had been changing; the IPK had diverged from its replicas by approximately 50 micrograms since their manufacture late in the 19th century. This led to several competing efforts to develop measurement technology precise enough to warrant replacing the kilogram artefact with a definition based directly on physical fundamental constants.
The International Committee for Weights and Measures (CIPM) approved a revision in November 2018 that defines the kilogram by defining the Planck constant to be exactly 6.62607015×10 kg⋅m⋅s, effectively defining the kilogram in terms of the second and the metre. The new definition took effect on 20 May 2019.
Prior to the redefinition, the kilogram and several other SI units based on the kilogram were defined by a man-made metal artifact: the Kilogramme des Archives from 1799 to 1889, and the IPK from 1889 to 2019.
In 1960, the metre, previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by krypton, and later the speed of light) so that the standard can be independently reproduced in different laboratories by following a written specification.
At the 94th Meeting of the CIPM in 2005, it was recommended that the same be done with the kilogram.
In October 2010, the CIPM voted to submit a resolution for consideration at the General Conference on Weights and Measures (CGPM), to "take note of an intention" that the kilogram be defined in terms of the Planck constant, h (which has dimensions of energy times time, thus mass × length / time) together with other physical constants. This resolution was accepted by the 24th conference of the CGPM in October 2011 and further discussed at the 25th conference in 2014. Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018. Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The Kibble balance is one way to do this.
As part of this project, a variety of very different technologies and approaches were considered and explored over many years. Some of these approaches were based on equipment and procedures that would enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and that expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. All approaches depend on converting a weight measurement to a mass and therefore require precise measurement of the strength of gravity in laboratories (gravimetry). All approaches would have precisely fixed one or more constants of nature at a defined value.
SI multiples
Main article: Orders of magnitude (mass) "Milligram" redirects here. For the American band, see Milligram (band). For the Serbian band, see Miligram (band). For the horse, see Milligram (horse).Because an SI unit may not have multiple prefixes (see SI prefix), prefixes are added to gram, rather than the base unit kilogram, which already has a prefix as part of its name. For instance, one-millionth of a kilogram is 1 mg (one milligram), not 1 μkg (one microkilogram).
| Submultiples | Multiples | ||||
|---|---|---|---|---|---|
| Value | SI symbol | Name | Value | SI symbol | Name |
| 10 g | dg | decigram | 10 g | dag | decagram |
| 10 g | cg | centigram | 10 g | hg | hectogram |
| 10 g | mg | milligram | 10 g | kg | kilogram |
| 10 g | μg | microgram | 10 g | Mg | megagram |
| 10 g | ng | nanogram | 10 g | Gg | gigagram |
| 10 g | pg | picogram | 10 g | Tg | teragram |
| 10 g | fg | femtogram | 10 g | Pg | petagram |
| 10 g | ag | attogram | 10 g | Eg | exagram |
| 10 g | zg | zeptogram | 10 g | Zg | zettagram |
| 10 g | yg | yoctogram | 10 g | Yg | yottagram |
| 10 g | rg | rontogram | 10 g | Rg | ronnagram |
| 10 g | qg | quectogram | 10 g | Qg | quettagram |
| Common prefixed units are in bold face. | |||||
Practical issues with SI weight names
- Serious medication errors have been made by confusing milligrams and micrograms when micrograms has been abbreviated. The abbreviation "mcg" rather than the SI symbol "μg" is formally mandated for medical practitioners in the US by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO). In the United Kingdom, the National Institute for Health and Care Excellence and Scottish Palliative Care Guidelines state that "micrograms" and "nanograms" must both be written in full, and never abbreviated as "mcg" or "μg".
- The hectogram (100 g) (Italian: ettogrammo or etto) is a very commonly used unit in the retail food trade in Italy.
- The former standard spelling and abbreviation "deka-" and "dk" produced abbreviations such as "dkm" (dekametre) and "dkg" (dekagram). As of 2020, the abbreviation "dkg" (10 g) is still used in parts of central Europe in retail for some foods such as cheese and meat.
- The unit name megagram is rarely used, and even then typically only in technical fields in contexts where especially rigorous consistency with the SI standard is desired. For most purposes, the name tonne is instead used. The tonne and its symbol, "t", were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. According to the BIPM, "This unit is sometimes referred to as 'metric ton' in some English-speaking countries."
See also
- Inertia – Fundamental principle of classical physics
- Kibble balance – Electromechanical weight measuring instrument
- Kilogram-force – Weight on earth of a one-kilogram mass
- Mass versus weight – Distinction between mass and weight
- Metric system – Decimal-based systems of measurement with 7 base units defined by physical constants
- National Institute of Standards and Technology – Measurement standards laboratory in the United States (NIST)
- Newton – Unit of force in physics
- Standard gravity – Standard gravitational acceleration on Earth
- Weight – Force on a mass due to gravity
Notes
- The avoirdupois pound is part of both United States customary system of units and the Imperial system of units. It is defined as exactly 0.45359237 kilograms.
- The French text (which is the authoritative text) states "Il n'est pas autorisé d'utiliser des abréviations pour les symboles et noms d'unités ..."
- Criterion: A combined total of at least five occurrences on the British National Corpus and the Corpus of Contemporary American English, including both the singular and the plural for both the -gram and the -gramme spelling.
References
- ^ "Kilogram". Oxford Dictionaries. Archived from the original on January 31, 2013. Retrieved November 3, 2011.
- "Kilogram". Collins Online Dictionary. Retrieved 14 October 2024.
- Merriam-Mebster definition of Kilo
- ^ International Bureau of Weights and Measures (20 May 2019), The International System of Units (SI) (PDF) (9th ed.), ISBN 978-92-822-2272-0, archived from the original on 18 October 2021
- "Mise en pratique for the definition of the kilogram in the SI". BIPM.org. 7 July 2021. Retrieved 18 February 2022.
- ^ Resnick, Brian (20 May 2019). "The new kilogram just debuted. It's a massive achievement". vox.com. Retrieved 23 May 2019.
- ^ Draft Resolution A "On the revision of the International System of units (SI)" to be submitted to the CGPM at its 26th meeting (2018) (PDF), archived (PDF) from the original on 2 April 2021
- Decision CIPM/105-13 (October 2016). The day is the 144th anniversary of the Metre Convention.
- SI Brochure: The International System of Units (SI). BIPM, 9th edition, 2019.
- The density of water is 0.999972 g/cm at 3.984 °C. See Franks, Felix (2012). The Physics and Physical Chemistry of Water. Springer. ISBN 978-1-4684-8334-5.
- Guyton; Lavoisier; Monge; Berthollet; et al. (1792). Annales de chimie ou Recueil de mémoires concernant la chimie et les arts qui en dépendent. Vol. 15–16. Paris: Chez Joseph de Boffe. p. 277.
- Gramme, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante
- ^ Zupko, Ronald Edward (1990). Revolution in Measurement: Western European Weights and Measures Since the Age of Science. Philadelphia: American Philosophical Society. ISBN 978-0-87169-186-6.
- "Treaty of the Metre". Encyclopædia Britannica. 2023. Retrieved 18 July 2023.
- ^ "Kilogram". Oxford English Dictionary. Oxford University Press. Retrieved 3 November 2011.
- Fowlers, HW; Fowler, FG (1964). The Concise Oxford Dictionary. Oxford: The Clarendon Press. Greek γράμμα (as it were γράφ-μα, Doric γράθμα) means "something written, a letter", but it came to be used as a unit of weight, apparently equal to 1/24 of an ounce (1/288 of a libra, which would correspond to about 1.14 grams in modern units), at some time during Late Antiquity. French gramme was adopted from Latin gramma, itself quite obscure, but found in the Carmen de ponderibus et mensuris (8.25) attributed by Remmius Palaemon (fl. 1st century), where it is the weight of two oboli (Charlton T. Lewis, Charles Short, A Latin Dictionary s.v. "gramma", 1879). Henry George Liddell. Robert Scott. A Greek-English Lexicon (revised and augmented edition, Oxford, 1940) s.v. γράμμα, citing the 10th-century work Geoponica and a 4th-century papyrus edited in L. Mitteis, Griechische Urkunden der Papyrussammlung zu Leipzig, vol. i (1906), 62 ii 27.
- "Décret relatif aux poids et aux mesures du 18 germinal an 3 (7 avril 1795)" [Decree of 18 Germinal, year III (April 7, 1795) regarding weights and measures]. Grandes lois de la République (in French). Digithèque de matériaux juridiques et politiques, Université de Perpignan. Retrieved 3 November 2011.
- Convention nationale, décret du 1 août 1793, ed. Duvergier, Collection complète des lois, décrets, ordonnances, règlemens avis du Conseil d'état, publiée sur les éditions officielles du Louvre, vol. 6 (2nd ed. 1834), p. 70. The metre (mètre) on which this definition depends was itself defined as the ten-millionth part of a quarter of Earth's meridian, given in traditional units as 3 pieds, 11.44 lignes (a ligne being the 12th part of a pouce (inch), or the 144th part of a pied.
- Peltier, Jean-Gabriel (1795). "Paris, during the year 1795". Monthly Review. 17: 556. Retrieved 2 August 2018. Contemporaneous English translation of the French decree of 1795
- "Spelling of "gram", etc". Weights and Measures Act 1985. Her Majesty's Stationery Office. 30 October 1985. Retrieved 6 November 2011.
- "kilo (n1)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved 8 November 2011.
- "kilo (n2)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved 8 November 2011.
- "Style Guide" (PDF). The Economist. 7 January 2002. Archived from the original (PDF) on 1 July 2017. Retrieved 8 November 2011.
- "kilogram, kg, kilo". Termium Plus. Government of Canada. 8 October 2009. Retrieved 29 May 2019.
- "kilo". How Many?. Archived from the original on 16 November 2011. Retrieved 6 November 2011.
-
29th Congress of the United States, Session 1 (13 May 1866). "H.R. 596, An Act to authorize the use of the metric system of weights and measures". Archived from the original on 5 July 2015.
{{cite web}}: CS1 maint: numeric names: authors list (link) -
"Metric System of Measurement:Interpretation of the International System of Units for the United States; Notice" (PDF). Federal Register. 63 (144): 40340. July 28, 1998. Archived from the original (PDF) on October 15, 2011. Retrieved November 10, 2011.
Obsolete Units As stated in the 1990 Federal Register notice, ...
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 130, ISBN 92-822-2213-6, archived (PDF) from the original on 4 June 2021, retrieved 16 December 2021
- Pallab Ghosh (16 November 2018). "Kilogram gets a new definition". BBC News. Retrieved 16 November 2018.
- International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 112, ISBN 92-822-2213-6, archived (PDF) from the original on 4 June 2021, retrieved 16 December 2021
- Recommendation 1: Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants (PDF). 94th meeting of the International Committee for Weights and Measures. October 2005. p. 233. Archived (PDF) from the original on 30 June 2007. Retrieved 7 February 2018.
- "NIST Backs Proposal for a Revamped System of Measurement Units". NIST. Nist.gov. 26 October 2010. Retrieved 3 April 2011.
- Ian Mills (29 September 2010). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). CCU. Retrieved 1 January 2011.
- Resolution 1 – On the possible future revision of the International System of Units, the SI (PDF). 24th meeting of the General Conference on Weights and Measures. Sèvres, France. 17–21 October 2011. Retrieved 25 October 2011.
- ^ "BIPM – Resolution 1 of the 25th CGPM". www.bipm.org. Retrieved 27 March 2017.
- "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. 23 October 2011. Retrieved 25 October 2011.
- Robinson, Ian A.; Schlamminger, Stephan (2016). "The watt or Kibble balance: A technique for implementing the new SI definition of the unit of mass". Metrologia. 53 (5): A46 – A74. Bibcode:2016Metro..53A..46R. doi:10.1088/0026-1394/53/5/A46. PMC 8752041. PMID 35023879.
- BIPM: SI Brochure: Section 3.2, The kilogram Archived March 29, 2016, at the Wayback Machine
- ^ "Prescribing Information for Liquid Medicines". Scottish Palliative Care Guidelines. Archived from the original on 10 July 2018. Retrieved 15 June 2015.
- "New Joint Commission "Do Not Use" List: Abbreviations, Acronyms, and Symbols". American Academy of Physical Medicine and Rehabilitation. Archived from the original on 15 September 2015. Retrieved 19 February 2024.
- "Prescription writing". National Institute for Health and Care Excellence. Retrieved 19 February 2024.
- Tom Stobart, The Cook's Encyclopedia, 1981, p. 525
- J.J. Kinder, V.M. Savini, Using Italian: A Guide to Contemporary Usage, 2004, ISBN 0521485568, p. 231
- Giacomo Devoto, Gian Carlo Oli, Nuovo vocabolario illustrato della lingua italiana, 1987, s.v. 'ètto': "frequentissima nell'uso comune: un e. di caffè, un e. di mortadella; formaggio a 2000 lire l'etto"
- U.S. National Bureau of Standards, The International Metric System of Weights and Measures, "Official Abbreviations of International Metric Units", 1932, p. 13
- "Jestřebická hovězí šunka 10 dkg | Rancherské speciality". eshop.rancherskespeciality.cz (in Czech). Archived from the original on 16 June 2020. Retrieved 16 June 2020.
- "Sedliacka šunka 1 dkg | Gazdovský dvor – Farma Busov Gaboltov". Sedliacka šunka 1 dkg (in Slovak). Archived from the original on 16 June 2020. Retrieved 16 June 2020.
- "sýr bazalkový – Farmářské Trhy". www.e-farmarsketrhy.cz (in Czech). Archived from the original on 16 June 2020. Retrieved 16 June 2020.
- "English Menu – Cafe Mediterran". Archived from the original on 16 June 2020. Retrieved 16 June 2020.
Beef steak 20 dkg; Beef steak 40 dkg;Thick crust 35 dkg
- "Termékek – Csíz Sajtműhely" (in Hungarian). Archived from the original on 16 June 2020. Retrieved 16 June 2020.
- Non-SI units that are accepted for use with the SI, SI Brochure: Section 4 (Table 8), BIPM
External links
- NIST Improves Accuracy of 'Watt Balance' Method for Defining the Kilogram
- The UK's National Physical Laboratory (NPL): Are any problems caused by having the kilogram defined in terms of a physical artefact? (FAQ – Mass & Density)
- NPL: NPL Kibble balance
- Metrology in France: Watt balance Archived March 19, 2014, at the Wayback Machine
- Australian National Measurement Institute: Redefining the kilogram through the Avogadro constant
- International Bureau of Weights and Measures (BIPM): Home page
- NZZ Folio: What a kilogram really weighs
- NPL: What are the differences between mass, weight, force and load?
- BBC: Getting the measure of a kilogram
- NPR: This Kilogram Has A Weight-Loss Problem, an interview with National Institute of Standards and Technology physicist Richard Steiner
- Avogadro and molar Planck constants for the redefinition of the kilogram
- Realization of the awaited definition of the kilogram
- Sample, Ian (9 November 2018). "In the balance: scientists vote on first change to kilogram in a century". The Guardian. Retrieved 9 November 2018.
Videos
- The BIPM – YouTube channel
- "The role of the Planck constant in physics" – presentation at 26th CGPM meeting at Versailles, France, November 2018 when voting on superseding the IPK took place on YouTube
| SI units | |
|---|---|
| Base units | |
| Derived units with special names | |
| Other accepted units | |
| See also | |
Categories: