| Revision as of 16:51, 2 July 2008 edit81.110.105.127 (talk) removed a whole bunch of info not relevant to diamondoid formation - added Mello & moldowan reference which proves diamondoids are derived from an original biotic carbon source← Previous edit | Latest revision as of 01:04, 19 July 2024 edit undoBoldLuis (talk | contribs)Extended confirmed users6,187 edits →See alsoTags: Mobile edit Mobile web edit Advanced mobile edit | ||
| (129 intermediate revisions by 79 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Various forms of carbon crystal lattices}} | |||
| A '''diamondoid''', in the context of building materials for ] components, most generally refers to structures that resemble ] in a broad sense: namely, strong, stiff structures containing dense, 3-D networks of ]s, formed chiefly from first and second row atoms with a ] of three or more. Examples of diamondoid structures would include crystalline ], ], and other stiff structures similar to diamond but with various atom substitutions which might include N, O, Si, S, and so forth. Sp²-] carbon structures that - in contrast to sp³-] carbon in ] - arrange in planar sheets ("]" sheets) are sometimes also included in the class of diamondoid materials for ], e.g., ], ] consisting of sheets of ] atoms rolled into tubes, spherical ] and other ] structures. | |||
| ⚫ | In chemistry, '''diamondoids''' are generalizations of the ] cage molecule known as ] (C<sub>10</sub>H<sub>16</sub>), the smallest unit cage structure of the ] ]. Diamondoids also known as '''nanodiamonds''' or '''condensed adamantanes''' may include one or more cages (adamantane, ], ], and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in ] deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule.<ref name="Dahl">{{cite journal |title= Isolation and Structure of Higher Diamondoids, Nanometer-Sized Diamond Molecules |first1= J. E. |last1= Dahl |first2= S. G. |last2= Liu |first3= R. M. K. |last3= Carlson |journal= ] |date= 3 January 2003 |volume= 299 |number= 5603 |pages= 96–99 |doi= 10.1126/science.1078239 |pmid=12459548|s2cid= 46688135 |doi-access= free }}</ref> These species are of interest as molecular approximations of the ] framework, terminated with C−H bonds. | ||
| == Chemistry == | |||
| ⚫ | In |
||
| ==Examples== | |||
| ] | ], ], ] and one isomer of ]]] | ||
| Examples include: | Examples include: | ||
| *] (C<sub>10</sub>H<sub>16</sub>) | * ] (C<sub>10</sub>H<sub>16</sub>) | ||
| * |
* ] (C<sub>12</sub>H<sub>18</sub>) | ||
| * |
* BC-8 (C<sub>14</sub>H<sub>20</sub>) | ||
| * |
* ] (C<sub>14</sub>H<sub>20</sub>) also ''diadamantane'', two face-fused cages | ||
| * |
* Triamantane (C<sub>18</sub>H<sub>24</sub>), also ''triadamantane''. Diamantane has four identical faces available for anchoring a new C<sub>4</sub>H<sub>4</sub> unit. | ||
| * |
* Isotetramantane (C<sub>22</sub>H<sub>28</sub>). Triamantane has eight faces on to which a new C<sub>4</sub>H<sub>4</sub> unit can be added resulting in four ]s. One of these isomers displays a helical twist and is therefore ]. The ] ]s have been separated. | ||
| * |
* Pentamantane has nine isomers with chemical formula C<sub>26</sub>H<sub>32</sub> and one more pentamantane exists with chemical formula C<sub>25</sub>H<sub>30</sub> | ||
| * Cyclohexamantane (C<sub>26</sub>H<sub>30</sub>)<ref>{{cite journal |first1=J. E. P. |last1=Dahl |first2=J. M. |last2=Moldowan |first3=T. M. |last3=Peakman |first4=J. C. |last4=Clardy |first5=E. |last5=Lobkovsky |first6=M. M. |last6=Olmstead |first7=P. W. |last7=May |first8=T. J. |last8=Davis |first9=J. W. |last9=Steeds |first10=K. E. |last10=Peters |first11=A. |last11=Pepper |first12=A. |last12=Ekuan |first13=R. M. K. |last13=Carlson | title= Isolation and Structural Proof of the Large Diamond Molecule, Cyclohexamantane (C<sub>26</sub>H<sub>30</sub>) | journal= Angewandte Chemie International Edition | year= 2003 | volume= 42 | pages= 2040–2044 |doi= 10.1002/anie.200250794 | pmid= 12746817 | issue= 18}}</ref> | |||
| ⚫ | * |
||
| *'''Super-adamantane''' (C<sub>35</sub>H<sub>36</sub>) | |||
| *] Be<sub>4</sub>O(O<sub>2</sub>CCH<sub>3</sub>)<sub>6</sub> | |||
| ⚫ | * Super-adamantane (C<sub>30</sub>H<sub>36</sub>) | ||
| One tetramantane isomer is the largest ever diamondoid prepared by ]. The first ever isolation of a wide range of diamondoids from petroleum took place in the following steps <ref name="Dahl"/>: a ] above 345 °C, the equivalent ], then ] at 400 to 450 °C in order to remove all non-diamondoid compounds <ref>Diamondoids are thermodynamically very stable and will survive this pyrolysis</ref> and then a series of ] separation techniques. | |||
| One tetramantane isomer is the largest ever diamondoid prepared by ] using a keto-] reaction to attach cyclopentane rings.<ref>{{cite journal | title=A New Approach to the Construction of Diamondoid Hydrocarbons. Synthesis of ''anti''-Tetramantane |last1=Burns |first1=W. |last2=McKervey |first2=M. A. |last3=Mitchell |first3=T. R. |last4=Rooney |first4=J. J. | journal= Journal of the American Chemical Society | volume=100 |issue=3 | pages=906–911 |year=1978 | doi=10.1021/ja00471a041}}</ref> Longer diamondoids have been formed from diamantane dicarboxylic acid.<ref>{{cite journal | journal= Angewandte Chemie International Edition | date= Mar 25, 2013 |volume=52 |issue=13 | pages=3717–3721 | title=Evidence of diamond nanowires formed inside carbon nanotubes from diamantane dicarboxylic acid |last1=Zhang |first1=J. |last2=Zhu |first2=Z. |last3=Feng |first3=Y. |last4=Ishiwata |first4=H. |last5=Miyata |first5=Y. |last6=Kitaura |first6=R. |last7=Dahl |first7=J. E. |last8=Carlson |first8=R. M. |last9=Fokina |first9=N. A. |last10=Schreiner |first10=P. R. |last11=Tománek |first11=D. |last12=Shinohara |first12=H. | pmid=23418054 | doi=10.1002/anie.201209192}}</ref> The first-ever isolation of a wide range of diamondoids from petroleum took place in the following steps:<ref name="Dahl"/> a ] above 345 °C, the equivalent ], then ] at 400 to 450 °C in order to remove all non-diamondoid compounds (diamondoids are thermodynamically very stable and will survive this pyrolysis) and then a series of ] separation techniques. | |||
| ⚫ | In one study a tetramantane compound is fitted with ] groups at the bridgehead positions |
||
| ⚫ | In one study a tetramantane compound is fitted with ] groups at the bridgehead positions.<ref>{{cite journal|title=Functionalized Nanodiamonds Part 3: Thiolation of Tertiary/Bridgehead Alcohols|first1= Boryslav A.|last1= Tkachenko|first2= Natalie A.|last2= Fokina|first3= Lesya V.|last3= Chernish|first4= Jeremy E. P.|last4= Dahl|first5= Shenggao|last5= Liu|first6= Robert M. K.|last6= Carlson|first7= Andrey A.|last7= Fokin|first8= Peter R.|last8= Schreiner|journal= Organic Letters |date=2006 |volume=8 |issue=9 |pages= 1767–70|doi=10.1021/ol053136g |pmid= 16623546}}</ref> This allows their anchorage to a ] surface and formation of ]s (diamond-on-gold). | ||
| ⚫ | Organic chemistry of diamondoids even extends to |
||
| ⚫ | Organic chemistry of diamondoids even extends to ''pentamantane''.<ref>{{cite journal | last1 = Fokin | first1 = Andrey A. | last2 = Schreiner | first2 = Peter R. | last3 = Fokina | first3 = Natalie A. | last4 = Tkachenko | first4 = Boryslav A. | last5 = Hausmann | first5 = Heike | last6 = Serafin | first6 = Michael | last7 = Dahl | first7 = Jeremy E. P. | last8 = Liu | first8 = Shenggao | last9 = Carlson | first9 = Robert M. K. | year = 2006 | title = Reactivity of Pentamantane (Td-Pentamantane): A Nanoscale Model of Diamond | journal = The Journal of Organic Chemistry | volume = 71 | issue = 22| pages = 8532–8540 | doi = 10.1021/jo061561x | pmid = 17064030 }}</ref> The medial position (base) in this molecule (the isomer pentamantane) is calculated to yield a more favorable ] than the apical position (top) and simple ] of pentamantane ''1'' with ] exclusively gives the medial bromo derivative ''2'' which on hydrolysis in water and ] forms the ] ''3''. | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | In contrast ] of |
||
| ⚫ | In contrast ] of ''1'' with ] gives the apical ] ''4'' as an intermediate which is hydrolysed to the apical ] ''5'' due to the higher ] of the active ] {{chem|NO|2|-}}{{chem|HNO|3|+}} species. This alcohol can react with ] to the bromide ''6'' and in a series of steps (not shown) to the corresponding ]. Pentamantane can also react with ] and ] bromide (TBABr) in a ] to the bromide but without selectivity. | ||
| ⚫ | == Origin and occurrence |
||
| ⚫ | == Origin and occurrence == | ||
| Diamondoids are found in ]s (oil and natural gas, mainly in condensates). Condensates, which are extremely light oils, have about a spoonful of diamondoids per gallon (about 3.78 liters).{{Fact|date=June 2008}} It has been suggested that diamondoids may come with the small amount of hydrocarbons that migrate from the ] to the ].{{Fact|date=June 2008}} In 2007, ] revealed that crude oil and the molecular diamondoids in them have an abiogenic mantle origin.<ref>http://aapg.confex.com/aapg/2007int/techprogram/A112905.htm</ref>. These findings have since been throughly debunked however by the Mello and Moldowan study of 2005 which clearly shows that diamondoids found in crude oil are derived from a biogenic carbon source.<ref>http://www.searchanddiscovery.net/documents/abstracts/2005research_calgary/abstracts/extended/mello/mello.htm</ref>. | |||
| Diamondoids are found in mature high-temperature ] fluids (volatile oils, condensates and wet gases). These fluids can have up to a spoonful of diamondoids per US gallon (3.78 liters). A review by Mello and Moldowan in 2005 showed that although the carbon in diamonds is not biological in origin, the diamondoids found in ] are composed of carbon from biological sources. This was determined by comparing the ratios of carbon ]s present.<ref>{{cite web|url=http://www.searchanddiscovery.net/documents/abstracts/2005research_calgary/abstracts/extended/mello/mello.htm|website=Search and Discovery|title=Petroleum: To Be Or Not To Be Abiogenic |first1=M. R. |last1=Mello |first2=J. M. |last2=Moldowan |date=2005}}</ref> | |||
| == Optical and electronic properties == | |||
| The ] for all diamondoids lies deep in the ] spectral region with optical ]s around 6 ]s and higher.<ref>{{cite journal |first1=L. |last1=Landt |first2=K. |last2=Klünder |first3=J. E. |last3=Dahl |first4=R. M. K. |last4=Carlson |first5=T. |last5=Möller |first6=C. |last6=Bostedt | title= Optical Response of Diamond Nanocrystals as a Function of Particle Size, Shape, and Symmetry | journal= Physical Review Letters | year= 2009 | volume= 103 |issue=4 | pages= 047402 |doi= 10.1103/PhysRevLett.103.047402 | bibcode=2009PhRvL.103d7402L | pmid=19659398|url=http://bib-pubdb1.desy.de/record/92624 }}</ref> The spectrum of each diamondoid is found to reflect its individual size, shape and ]. Due to their well-defined size and structure diamondoids also serve as a model system for electronic structure calculations.<ref>{{cite journal |first1=M. |last1=Vörös |first2=A. |last2=Gali | title= Optical absorption of diamond nanocrystals from ''ab initio'' density-functional calculations | journal= Physical Review B | year= 2009 | volume= 80 |issue=16 | pages= 161411 |doi= 10.1103/PhysRevB.80.161411|bibcode = 2009PhRvB..80p1411V }}</ref> | |||
| Many of the optoelectronic properties of diamondoids are determined by the difference in the nature of the ]: the former is a ], whereas the latter is a ]. As a result, the energy of the lowest unoccupied molecular orbital is roughly independent of the size of the diamondoid.<ref name="dmc_diamondoid">{{cite journal |first1=N. D. |last1=Drummond |first2=A. J. |last2=Williamson |first3=R. J. |last3=Needs |first4=G. |last4=Galli | title= Electron emission from diamondoids: a diffusion quantum Monte Carlo study | journal= Physical Review Letters | year= 2005 | volume= 95 |issue=9 | pages= 096801–096804 |doi= 10.1103/PhysRevLett.95.096801 | bibcode=2005PhRvL..95i6801D|arxiv = 0801.0381 | pmid=16197235|s2cid=16703233 }}</ref><ref>{{cite journal |first1=T. M. |last1=Willey |first2=C. |last2=Bostedt |first3=T. |last3=van Buuren |first4=J. E. |last4=Dahl |first5=S. G. |last5=Liu |first6=R. M. K. |last6=Carlson |first7=L. J. |last7=Terminello |first8=T. |last8=Möller | title= Molecular Limits to the Quantum Confinement Model in Diamond Clusters | journal= Physical Review Letters | year= 2005 | volume= 95 |issue=11 | pages= 113401–113404 |doi= 10.1103/PhysRevLett.95.113401 | bibcode=2005PhRvL..95k3401W | pmid=16197003|url=https://digital.library.unt.edu/ark:/67531/metadc876588/ |type=Submitted manuscript }}</ref> | |||
| Diamondoids have been found to exhibit a negative ], making them potentially useful in ] devices.<ref name="dmc_diamondoid" /><ref>{{cite journal |first1=W. L. |last1=Yang |first2=J. D. |last2=Fabbri |first3=T. M. |last3=Willey |first4=J. R. I. |last4=Lee |first5=J. E. |last5=Dahl |first6=R. M. K. |last6=Carlson |first7=P. R. |last7=Schreiner |first8=A. A. |last8=Fokin |first9=B. A. |last9=Tkachenko |first10=N. A. |last10=Fokina |first11=W. |last11=Meevasana |first12=N. |last12=Mannella |first13=K. |last13=Tanaka |first14=X.-J. |last14=Zhou |first15=T. |last15=van Buuren |first16=M. A. |last16=Kelly |first17=Z. |last17=Hussain |first18=N. A. |last18=Melosh |first19=Z.-X. |last19=Shen | title= Monochromatic Electron Photoemission from Diamondoid Monolayers | journal= Science | year= 2007 | volume= 316 |issue=5830 | pages= 1460–1462 |doi= 10.1126/science.1141811 |pmid=17556579 |bibcode = 2007Sci...316.1460Y |url=https://cloudfront.escholarship.org/dist/prd/content/qt5h79b9nr/qt5h79b9nr.pdf |doi-access=free }}</ref> | |||
| ==See also== | ==See also== | ||
| * Other diamond-like compounds: ] | * Other diamond-like compounds: ] | ||
| * ] | |||
| * ] | |||
| ⚫ | == |
||
| ⚫ | * | ||
| ⚫ | * | ||
| ⚫ | * | ||
| ⚫ | * | ||
| ==References== | ==References== | ||
| {{reflist|30em}} | |||
| <references/> | |||
| ⚫ | ==External links== | ||
| ] | |||
| ⚫ | * | ||
| ] | |||
| ⚫ | * | ||
| ⚫ | * | ||
| ⚫ | * | ||
| * | |||
| * | |||
| * | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 01:04, 19 July 2024
Various forms of carbon crystal latticesIn chemistry, diamondoids are generalizations of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds.
Examples

Examples include:
- Adamantane (C10H16)
- Iceane (C12H18)
- BC-8 (C14H20)
- Diamantane (C14H20) also diadamantane, two face-fused cages
- Triamantane (C18H24), also triadamantane. Diamantane has four identical faces available for anchoring a new C4H4 unit.
- Isotetramantane (C22H28). Triamantane has eight faces on to which a new C4H4 unit can be added resulting in four isomers. One of these isomers displays a helical twist and is therefore prochiral. The P and M enantiomers have been separated.
- Pentamantane has nine isomers with chemical formula C26H32 and one more pentamantane exists with chemical formula C25H30
- Cyclohexamantane (C26H30)
- Super-adamantane (C30H36)
One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis using a keto-carbenoid reaction to attach cyclopentane rings. Longer diamondoids have been formed from diamantane dicarboxylic acid. The first-ever isolation of a wide range of diamondoids from petroleum took place in the following steps: a vacuum distillation above 345 °C, the equivalent atmospheric boiling point, then pyrolysis at 400 to 450 °C in order to remove all non-diamondoid compounds (diamondoids are thermodynamically very stable and will survive this pyrolysis) and then a series of high-performance liquid chromatography separation techniques.
In one study a tetramantane compound is fitted with thiol groups at the bridgehead positions. This allows their anchorage to a gold surface and formation of self-assembled monolayers (diamond-on-gold).
Organic chemistry of diamondoids even extends to pentamantane. The medial position (base) in this molecule (the isomer pentamantane) is calculated to yield a more favorable carbocation than the apical position (top) and simple bromination of pentamantane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water and DMF forms the alcohol 3.
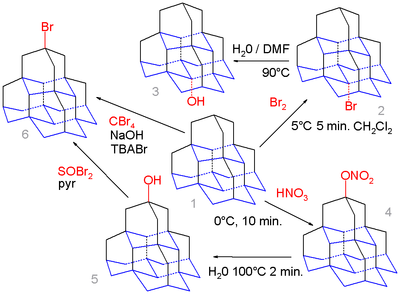
In contrast nitrooxylation of 1 with nitric acid gives the apical nitrate 4 as an intermediate which is hydrolysed to the apical alcohol 5 due to the higher steric demand of the active electrophilic NO
2HNO
3 species. This alcohol can react with thionyl bromide to the bromide 6 and in a series of steps (not shown) to the corresponding thiol. Pentamantane can also react with tetrabromomethane and tetra-n-butylammonium bromide (TBABr) in a free radical reaction to the bromide but without selectivity.
Origin and occurrence
Diamondoids are found in mature high-temperature petroleum fluids (volatile oils, condensates and wet gases). These fluids can have up to a spoonful of diamondoids per US gallon (3.78 liters). A review by Mello and Moldowan in 2005 showed that although the carbon in diamonds is not biological in origin, the diamondoids found in petroleum are composed of carbon from biological sources. This was determined by comparing the ratios of carbon isotopes present.
Optical and electronic properties
The optical absorption for all diamondoids lies deep in the ultraviolet spectral region with optical band gaps around 6 electronvolts and higher. The spectrum of each diamondoid is found to reflect its individual size, shape and symmetry. Due to their well-defined size and structure diamondoids also serve as a model system for electronic structure calculations.
Many of the optoelectronic properties of diamondoids are determined by the difference in the nature of the highest occupied and lowest unoccupied molecular orbitals: the former is a bulk state, whereas the latter is a surface state. As a result, the energy of the lowest unoccupied molecular orbital is roughly independent of the size of the diamondoid.
Diamondoids have been found to exhibit a negative electron affinity, making them potentially useful in electron-emission devices.
See also
- Other diamond-like compounds: Boron nitride
- Abiogenic petroleum origin
- Nanorobot
References
- ^ Dahl, J. E.; Liu, S. G.; Carlson, R. M. K. (3 January 2003). "Isolation and Structure of Higher Diamondoids, Nanometer-Sized Diamond Molecules". Science. 299 (5603): 96–99. doi:10.1126/science.1078239. PMID 12459548. S2CID 46688135.
- Dahl, J. E. P.; Moldowan, J. M.; Peakman, T. M.; Clardy, J. C.; Lobkovsky, E.; Olmstead, M. M.; May, P. W.; Davis, T. J.; Steeds, J. W.; Peters, K. E.; Pepper, A.; Ekuan, A.; Carlson, R. M. K. (2003). "Isolation and Structural Proof of the Large Diamond Molecule, Cyclohexamantane (C26H30)". Angewandte Chemie International Edition. 42 (18): 2040–2044. doi:10.1002/anie.200250794. PMID 12746817.
- Burns, W.; McKervey, M. A.; Mitchell, T. R.; Rooney, J. J. (1978). "A New Approach to the Construction of Diamondoid Hydrocarbons. Synthesis of anti-Tetramantane". Journal of the American Chemical Society. 100 (3): 906–911. doi:10.1021/ja00471a041.
- Zhang, J.; Zhu, Z.; Feng, Y.; Ishiwata, H.; Miyata, Y.; Kitaura, R.; Dahl, J. E.; Carlson, R. M.; Fokina, N. A.; Schreiner, P. R.; Tománek, D.; Shinohara, H. (Mar 25, 2013). "Evidence of diamond nanowires formed inside carbon nanotubes from diamantane dicarboxylic acid". Angewandte Chemie International Edition. 52 (13): 3717–3721. doi:10.1002/anie.201209192. PMID 23418054.
- Tkachenko, Boryslav A.; Fokina, Natalie A.; Chernish, Lesya V.; Dahl, Jeremy E. P.; Liu, Shenggao; Carlson, Robert M. K.; Fokin, Andrey A.; Schreiner, Peter R. (2006). "Functionalized Nanodiamonds Part 3: Thiolation of Tertiary/Bridgehead Alcohols". Organic Letters. 8 (9): 1767–70. doi:10.1021/ol053136g. PMID 16623546.
- Fokin, Andrey A.; Schreiner, Peter R.; Fokina, Natalie A.; Tkachenko, Boryslav A.; Hausmann, Heike; Serafin, Michael; Dahl, Jeremy E. P.; Liu, Shenggao; Carlson, Robert M. K. (2006). "Reactivity of Pentamantane (Td-Pentamantane): A Nanoscale Model of Diamond". The Journal of Organic Chemistry. 71 (22): 8532–8540. doi:10.1021/jo061561x. PMID 17064030.
- Mello, M. R.; Moldowan, J. M. (2005). "Petroleum: To Be Or Not To Be Abiogenic". Search and Discovery.
- Landt, L.; Klünder, K.; Dahl, J. E.; Carlson, R. M. K.; Möller, T.; Bostedt, C. (2009). "Optical Response of Diamond Nanocrystals as a Function of Particle Size, Shape, and Symmetry". Physical Review Letters. 103 (4): 047402. Bibcode:2009PhRvL.103d7402L. doi:10.1103/PhysRevLett.103.047402. PMID 19659398.
- Vörös, M.; Gali, A. (2009). "Optical absorption of diamond nanocrystals from ab initio density-functional calculations". Physical Review B. 80 (16): 161411. Bibcode:2009PhRvB..80p1411V. doi:10.1103/PhysRevB.80.161411.
- ^ Drummond, N. D.; Williamson, A. J.; Needs, R. J.; Galli, G. (2005). "Electron emission from diamondoids: a diffusion quantum Monte Carlo study". Physical Review Letters. 95 (9): 096801–096804. arXiv:0801.0381. Bibcode:2005PhRvL..95i6801D. doi:10.1103/PhysRevLett.95.096801. PMID 16197235. S2CID 16703233.
- Willey, T. M.; Bostedt, C.; van Buuren, T.; Dahl, J. E.; Liu, S. G.; Carlson, R. M. K.; Terminello, L. J.; Möller, T. (2005). "Molecular Limits to the Quantum Confinement Model in Diamond Clusters". Physical Review Letters (Submitted manuscript). 95 (11): 113401–113404. Bibcode:2005PhRvL..95k3401W. doi:10.1103/PhysRevLett.95.113401. PMID 16197003.
- Yang, W. L.; Fabbri, J. D.; Willey, T. M.; Lee, J. R. I.; Dahl, J. E.; Carlson, R. M. K.; Schreiner, P. R.; Fokin, A. A.; Tkachenko, B. A.; Fokina, N. A.; Meevasana, W.; Mannella, N.; Tanaka, K.; Zhou, X.-J.; van Buuren, T.; Kelly, M. A.; Hussain, Z.; Melosh, N. A.; Shen, Z.-X. (2007). "Monochromatic Electron Photoemission from Diamondoid Monolayers" (PDF). Science. 316 (5830): 1460–1462. Bibcode:2007Sci...316.1460Y. doi:10.1126/science.1141811. PMID 17556579.
External links
- Cluster and Nanocrystal Research Group, Technische Universität Berlin
- Molecular Diamond Technologies, Chevron Texaco
- Nanotechnology and the arrival of the Diamond Age
- Laser Raman Spectroscopy and Modelling of Diamondoids
- Electronic and Optical Properties of Diamondoids (free download)
- Diamondoid Molecules: With Applications in Biomedicine, Materials Science, Nanotechnology & Petroleum Science
- Diamondoid-functionalized gold nanogaps as sensors for natural, mutated, and epigenetically modified DNA nucleotides