| Revision as of 05:59, 9 January 2006 editYurikBot (talk | contribs)278,165 editsm robot Adding: es← Previous edit | Latest revision as of 16:24, 29 December 2024 edit undoOzzie10aaaa (talk | contribs)Autopatrolled, Extended confirmed users, New page reviewers213,612 edits →Education and self-management | ||
| (753 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Chronic loss of kidney function}} | |||
| {{DiseaseDisorder infobox | | |||
| {{Infobox medical condition (new) | |||
| Name = Diabetic nephropathy | | |||
| |
| name = Diabetic nephropathy | ||
| |
| synonyms = Diabetic kidney disease | ||
| | image = Nodular_glomerulosclerosis.jpeg | |||
| | caption = Two glomeruli in diabetic nephropathy: the acellular light purple areas within the capillary tufts are the destructive mesangial matrix deposits. | |||
| | pronounce = | |||
| | field = | |||
| | symptoms = ],<ref name="dmsj2019">{{cite journal |last1=Alamo |first1=A. |last2=Campagna |first2=D. |last3=Di Pino |first3=A. |last4=Russo |first4=C. |last5=Calogero |first5=A. E. |last6=Polosa |first6=R. |last7=Purrello |first7=F. |date=October 2019 |title=Smoking and diabetes: dangerous liaisons and confusing relationships |url=https://dmsjournal.biomedcentral.com/track/pdf/10.1186/s13098-019-0482-2.pdf |journal=Diabetology & Metabolic Syndrome |publisher=] |volume=11 |issue=85 |page=85 |doi=10.1186/s13098-019-0482-2 |doi-access=free |issn=1758-5996 |pmc=6813988 |pmid=31666811 |s2cid=204882089 |access-date=20 August 2021}}</ref> ],<ref name="dmsj2019"/> high blood pressure,<ref name="dmsj2019"/> tiredness<ref name="nih22"/> | |||
| | complications = | |||
| | onset = | |||
| | duration = | |||
| | types = | |||
| | causes = | |||
| | risks = High blood pressure, ],<ref name="dmsj2019"/> unstable blood glucose<ref name="nih22"/> | |||
| | diagnosis = Abnormal levels of urinary albumin<ref name="lewis2014">{{cite journal | vauthors = Lewis G, Maxwell AP | title = Risk factor control is key in diabetic nephropathy | journal = The Practitioner | volume = 258 | issue = 1768 | pages = 13–7, 2 | date = February 2014 | pmid = 24689163 }}</ref> | |||
| | differential = | |||
| | prevention = ]<ref name="dmsj2019"/> | |||
| | treatment = ACE inhibitors<ref name="ace">{{cite journal | vauthors = Lim AK | title = Diabetic nephropathy – complications and treatment | journal = ] | volume = 7 | pages = 361–81 | date = 2014 | pmid = 25342915 | pmc = 4206379 | doi = 10.2147/IJNRD.S40172 | doi-access = free }}</ref> | |||
| | medication = | |||
| | prognosis = | |||
| | frequency = | |||
| | deaths = | |||
| }} | }} | ||
| ] | |||
| '''Diabetic nephropathy''' (''nephropatia diabetica''), also known as '''Kimmelstiel-Wilson syndrome''' and '''intercapillary glomerulonephritis''', is a progressive ] caused by ] of ] in the ] ]. It is characterized by nodular ]. It is due to longstanding ], and is a prime cause for ] in many Western countries. | |||
| '''Diabetic nephropathy''', also known as '''diabetic kidney disease''',<ref>{{cite book|title=Nutrition Therapy for Chronic Kidney Disease|vauthors=Kittell F|publisher=CRC Press|year=2012|veditors=Thomas LK, Othersen JB|page=198|chapter=Diabetes Management|isbn=9781439849491|chapter-url=https://books.google.com/books?id=DreAtq_hKmcC&pg=PA198}}</ref> is the chronic loss of ] occurring in those with ]. Diabetic nephropathy is the leading causes of ] (CKD) and end-stage renal disease (ESRD) globally. The triad of protein leaking into the urine (proteinuria or albuminuria), rising blood pressure with hypertension and then falling renal function is common to many forms of CKD. ] due to damage of the ] may become massive, and cause a low ] with resulting ] so called ]. Likewise, the estimated ] (eGFR) may progressively fall from a normal of over 90 ml/min/1.73m<sup>2</sup> to less than 15, at which point the patient is said to have ].<ref>{{cite book|last1=Longo|first1=Dan|title=Harrison's manual of medicine|last2=Fauci|first2=Anthony|last3=Kasper|first3=Dennis|last4=Hauser|first4=Stephen|last5=Jameson|first5=J.|last6=Loscalzo|first6=Joseph|date=2013|publisher=McGraw-Hill Medical|isbn=978-0-07-174519-2|edition=18th|location=New York|page=2982|name-list-style=vanc}}</ref> It usually is slowly progressive over years.<ref>{{cite journal|vauthors=Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH|date=August 2016|title=Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014|journal=JAMA|volume=316|issue=6|pages=602–10|doi=10.1001/jama.2016.10924|pmc=5444809|pmid=27532915}}</ref> | |||
| ==History== | |||
| The syndromed was discovered by ] ] ] (1906-1997) and ]-born ] physician ] (1900-1970) and was published for the first time in ]. | |||
| Pathophysiologic abnormalities in diabetic nephropathy usually begin with long-standing poorly controlled blood glucose levels. This is followed by multiple changes in the filtration units of the kidneys, the ]s. (There are normally about 750,000–1.5 million nephrons in each adult kidney).<ref>{{cite book|last1=Hall|first1=John|title=Textbook of Medical Physiology|last2=Guyton|first2=Arthur|date=2005|publisher=W.B. Saunders|isbn=978-0-7216-0240-0|edition=11th|location=Philadelphia|page=310|name-list-style=vanc}}</ref> Initially, there is constriction of the ] and dilation of ], with resulting glomerular capillary hypertension and hyperfiltration particularly as nephrons become obsolescent and the adaption of hyperfiltration paradoxically causes further ] related damage to the delicate glomerular capillaries, further proteinuria, rising blood pressure and a vicious circle of additional nephron damage and decline in overall renal function.<ref>{{Cite journal |last1=Hostetter |first1=T. H. |last2=Olson |first2=J. L. |last3=Rennke |first3=H. G. |last4=Venkatachalam |first4=M. A. |last5=Brenner |first5=B. M. |date=July 1981 |title=Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation |url=https://pubmed.ncbi.nlm.nih.gov/7246778/ |journal=The American Journal of Physiology |volume=241 |issue=1 |pages=F85–93 |doi=10.1152/ajprenal.1981.241.1.F85 |issn=0002-9513 |pmid=7246778|s2cid=1553863 }}</ref><ref>{{cite web|title=diabetic nephropathy|url=http://medical-dictionary.thefreedictionary.com/diabetic+nephropathy|access-date=2015-06-27}}</ref> Concurrently, there are changes within the glomerulus itself: these include a thickening of the ], a widening of the slit membranes of the ], an increase in the number of ]s, and an increase in mesangial matrix. This matrix invades the glomerular capillaries and produces deposits called Kimmelstiel-Wilson nodules. The mesangial cells and matrix can progressively expand and consume the entire glomerulus, shutting off filtration.<ref name="Schlöndorff_20092">{{cite journal|vauthors=Schlöndorff D, Banas B|date=June 2009|title=The mesangial cell revisited: no cell is an island|journal=Journal of the American Society of Nephrology|volume=20|issue=6|pages=1179–87|doi=10.1681/ASN.2008050549|pmid=19470685|doi-access=free}}</ref><!--Prevention and Treatment--> | |||
| ==Epidemiology== | |||
| The syndrome can be seen in patients with ] ] (15 years or more after onset), so patients are usually of older age (between 50 and 70 years old). The disease is progressive and may cause ] two or three years after the initial lesions. and is more frequent in women. Diabetic nephropathy is the most common cause of chronic kidney failure and end-stage kidney disease in the United States. People with both type 1 and type 2 diabetes are at risk. The risk is higher if blood-glucose levels are poorly controlled. However, once nephropathy develops, the greatest rate of progression is seen in patients with poor control of their blood pressure. | |||
| The status of diabetic nephropathy may be monitored by measuring two values: the amount of protein in the urine - ]; and a blood test called the serum ]. The amount of the proteinuria reflects the degree of damage to any still-functioning glomeruli. The value of the ] can be used to calculate the ] (eGFR), which reflects the percentage of glomeruli which are no longer filtering the blood. {{citation needed|date=December 2017}} Treatment with an ] or ], which dilates the ] exiting the glomerulus, thus reducing the ] within the glomerular capillaries, may slow (but not stop) progression of the disease. Three classes of diabetes medications – ]s, ]s, and ]s– are also thought to slow the progression of diabetic nephropathy.<ref>{{cite journal|vauthors=de Boer IH|date=August 2017|title=A New Chapter for Diabetic Kidney Disease|journal=The New England Journal of Medicine|volume=377|issue=9|pages=885–887|doi=10.1056/nejme1708949|pmid=28854097}}</ref><!--Epidemiology--> | |||
| ==Etiopathology== | |||
| The earliest detectable change in the course of diabetic nephropathy is a thickening in the glomerulus. At this stage, the kidney may start allowing more ] (plasma protein) than normal in the ] (]), and this can be detected by sensitive ]s for albumin. This stage is called "microabuminuria". It can appear 5 to 10 years before other symptoms develop. As diabetic nephropathy progresses, increasing numbers of glomeruli are destroyed by nodular glomerulosclerosis. Now the amounts of albumin being excreted in the urine increases, and may be detected by ordinary ] techniques. At this stage, a kidney ] clearly shows diabetic nephropathy. | |||
| Diabetic nephropathy is the most common cause of end-stage renal disease and is a serious complication that affects approximately one quarter of adults with diabetes in the United States.<ref name="Fernandez201422">{{cite journal|vauthors=Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF|date=September 2014|title=Diabetic kidney disease: from physiology to therapeutics|journal=The Journal of Physiology|volume=592|issue=18|pages=3997–4012|doi=10.1113/jphysiol.2014.272328|pmc=4198010|pmid=24907306}}</ref><ref name="Ding201522">{{cite journal|vauthors=Ding Y, Choi ME|date=January 2015|title=Autophagy in diabetic nephropathy|journal=The Journal of Endocrinology|volume=224|issue=1|pages=R15–30|doi=10.1530/JOE-14-0437|pmc=4238413|pmid=25349246}}</ref> Affected individuals with end-stage kidney disease often require ] and eventually ] to replace the failed kidney function.<ref name="ReferenceB22">{{cite journal|vauthors=Lizicarova D, Krahulec B, Hirnerova E, Gaspar L, Celecova Z|year=2014|title=Risk factors in diabetic nephropathy progression at present|journal=Bratislavske Lekarske Listy|volume=115|issue=8|pages=517–21|doi=10.4149/BLL_2014_101|pmid=25246291|doi-access=free}}</ref> Diabetic nephropathy is associated with an increased ] in general, particularly from ].<ref name="Fernandez201422"/><ref name="Pálsson R 201422">{{cite journal|vauthors=Pálsson R, Patel UD|date=May 2014|title=Cardiovascular complications of diabetic kidney disease|journal=Advances in Chronic Kidney Disease|volume=21|issue=3|pages=273–80|doi=10.1053/j.ackd.2014.03.003|pmc=4045477|pmid=24780455}}</ref> | |||
| ==Signs and symptoms== | ==Signs and symptoms== | ||
| The onset of symptoms is 5 to 10 years after the disease begins.<ref name="nih22">{{Cite web|title=Diabetes and kidney disease: MedlinePlus Medical Encyclopedia|url=https://www.nlm.nih.gov/medlineplus/ency/article/000494.htm|website=www.nlm.nih.gov|access-date=2015-06-27}}</ref> A usual first symptom is frequent urination at night: ]. Other symptoms include ], ]s, a ], ], ], frequent daytime urination, ], ], and ].<ref name="nih22"/> The clinical presentation of diabetic nephropathy (DN) is characterized by proteinuria (protein in the urine), hypertension and progressive loss of kidney function. The process may be initially indolent, making regular screening for diabetic nephropathy in patients with diabetes mellitus of great importance.<ref>{{Cite journal|last=Kussman|first=M. J.|date=1976-10-18|title=The clinical course of diabetic nephropathy|journal=Journal of the American Medical Association|volume=236|issue=16|pages=1861–1863|doi=10.1001/jama.236.16.1861|pmid=989537|issn=0098-7484}}</ref> | |||
| Kidney failure provoked by glomerulosclerosis lead to fluid filtration deficits and other disorders of kidney function. There is an increase in ] (]) and of fluid retention in the body (]). Other ] may be ] of the ] and ] (nephrotic syndrome). | |||
| ==Risk factors== | |||
| Throughout its early course, diabetic nephropathy has no ]s. They develop in late stages and may be a result of excretion of high amounts of protein in the urine or due to renal failure: | |||
| Not all patients with diabetes go on to develop diabetic nephropathy. The main risk factors that increase the likelihood of developing diabetic nephropathy are:<ref name="nih22"/> | |||
| * Poor control of blood glucose | |||
| * Uncontrolled ] | |||
| * ], with onset before age 20 | |||
| * Past or current ]<ref>{{cite journal|vauthors=Jiang N|title=Smoking and the risk of diabetic nephropathy in patients with type 1 and type 2 diabetes: a meta-analysis of observational studies | |||
| |journal=Oncotarget|date=Nov 2017|volume=8|issue =54|pages=93209–93218 | |||
| |doi=10.18632/oncotarget.21478|pmid=29190990|pmc=5696256 | |||
| }}</ref> | |||
| * A family history of diabetic nephropathy- certain genes have been identified that are associated with DN. ( However, no direct correlation has been established yet.<ref>{{Cite journal|last1=Freedman|first1=Barry I.|last2=Bostrom|first2=Meredith|last3=Daeihagh|first3=Pirouz|last4=Bowden|first4=Donald W.|date=2007-10-17|title=Genetic Factors in Diabetic Nephropathy|journal=Clinical Journal of the American Society of Nephrology|volume=2|issue=6|pages=1306–1316|doi=10.2215/cjn.02560607|pmid=17942768|issn=1555-9041|doi-access=free}}</ref> One of these genes is APOL1, which has been found to be associated with nephropathy in African American individuals.<ref>{{Cite journal|last1=Kruzel-Davila|first1=Etty|last2=Wasser|first2=Walter G.|last3=Aviram|first3=Sharon|last4=Skorecki|first4=Karl|date=March 2016|title=APOL1 nephropathy: from gene to mechanisms of kidney injury|journal=Nephrology, Dialysis, Transplantation|volume=31|issue=3|pages=349–358|doi=10.1093/ndt/gfu391|issn=1460-2385|pmid=25561578|doi-access=free}}</ref>) | |||
| * Certain racial groups (African Americans, Mexican Americans, and Pima Indians are at higher risk). | |||
| ==Pathophysiology== | |||
| * ]: swelling, usually around the ]s in the mornings; later, general body swelling may result, such as swelling of the legs | |||
| ] | |||
| * foamy appearance or excessive frothing of the urine | |||
| {{See also|Renal physiology|l1=Review of normal Renal Physiology}} | |||
| * unintentional weight gain (from fluid accumulation) | |||
| * ] (poor appetite) | |||
| * ] and ] | |||
| * ] (general ill feeling) | |||
| * ] | |||
| * ] | |||
| * frequent ]s | |||
| * generalized ] | |||
| The disease progression of diabetic nephropathy involves various clinical stages: hyperfiltration, microalbuminuria, macroalbuminuria, nephrotic proteinuria to progressive chronic kidney disease leading to end-stage renal disease (ESRD). The damage is exerted on all compartments of the kidney: the glomerulus, the renal tubules, the vasculature (afferent and efferent renal arterioles) and the interstitium. Renal fibrosis is the final common pathway of DN. This fibrosis is a product of multiple mechanisms including renal hemodynamic changes, glucose metabolism abnormalities associated with oxidative stress as well as inflammatory processes and an overactive ] (RAAS).{{citation needed|date=May 2020}} | |||
| The first laboratory abnormality is a positive microalbuminuria test. Most often, the diagnosis is suspected when a routine urinalysis of a person with diabetes shows too much protein in the urine (proteinuria). The urinalysis may also show ] in the urine, especially if blood glucose is poorly controlled. Serum ] and ] may increase as kidney damage progresses. | |||
| The pathophysiology of diabetic nephropathy is thought to involve an interaction between hemodynamic and metabolic factors.<ref>{{Cite journal|last1=Lin|first1=Yi-Chih|last2=Chang|first2=Yu-Hsing|last3=Yang|first3=Shao-Yu|last4=Wu|first4=Kwan-Dun|last5=Chu|first5=Tzong-Shinn|date=2018-08-01|title=Update of pathophysiology and management of diabetic kidney disease|journal=Journal of the Formosan Medical Association|language=en|volume=117|issue=8|pages=662–675|doi=10.1016/j.jfma.2018.02.007|pmid=29486908|issn=0929-6646|doi-access=free}}</ref> | |||
| A kidney ] confirms the diagnosis, although it is not always necessary if the case is straightforward, with a documented progression of proteinuria over time and presence of diabetic ] on examination of the ] of the ]s. | |||
| Hemodynamic factors include an increase in systemic and intraglomerular pressure, as well as the over-activation of the RAAS. Studies have shown that in the setting of diabetes, various factors stimulate the RAAS, which is one of the most important pathways in diabetic nephropathy pathophysiology. Due to the higher load of filtered glucose, there is an up-regulation in the sodium-glucose cotransporter 2 (SGLT2) in the proximal tubules, which cotransports sodium and glucose back into circulation. This leads to a decrease in the delivery of sodium chloride to the macula densa in the distal tubules, promoting the release of renin and over-activating RAAS.<ref>{{Citation|last1=Anderson|first1=Sharon|title=Pathogenesis of Diabetic Glomerulopathy: The Role of Glomerular Hyperfiltration|date=1988|work=The Kidney and Hypertension in Diabetes Mellitus|pages=139–146|publisher=Springer US|isbn=978-1-4757-1976-5|last2=Brenner|first2=Barry M.|doi=10.1007/978-1-4757-1974-1_17}}</ref> Hyperfiltration is one of the earliest features of DN. Several mechanisms have been proposed to cause hyperfiltration. One of these mechanisms is that as glomeruli becomes hypertrophied, filtration surface area initially increases. Another possible mechanism is that abnormal vascular control in diabetic nephropathy leads to a reduction in afferent glomerular arteriolar resistance and an increase in efferent glomerular arteriolar resistance, leading to a net increase in renal blood flow (RBF) and glomerular filtration rate (GFR).<ref>{{Cite journal|last=Hostetter|first=Thomas H.|date=March 2003|title=Hyperfiltration and glomerulosclerosis|url=https://pubmed.ncbi.nlm.nih.gov/12704579/|journal=Seminars in Nephrology|volume=23|issue=2|pages=194–199|doi=10.1053/anep.2003.50017|issn=0270-9295|pmid=12704579}}</ref> Glomerular hyperfiltration and an aberrant regulation of RAAS lead to increased intraglomerular pressure, causing stress on the endothelial cells, the mesangial cells and the podocytes. This exacerbates the dysfunction caused by the metabolic effects of hyperglycemia.{{citation needed|date=May 2020}} | |||
| ==Treatment== | |||
| The goals of treatment are to slow the progression of kidney damage and control related complications. The main treatment, once proteinuria is established, is ] drugs, which usually reduces proteinuria levels and slows the progression of diabetic nephropathy. Many studies have shown that related drugs, ]s (ARBs), have a similar benefit. In fact, a combination may be best. | |||
| Metabolic factors include the formation of ]s (AGEs), which have a central role in the pathophysiology of many of the complications of diabetes mellitus, including cardiovascular complications.<ref>{{Cite journal|last1=Soldatos|first1=G.|last2=Cooper|first2=M. E.|date=2008-11-13|title=Diabetic nephropathy: Important pathophysiologic mechanisms|url=http://www.sciencedirect.com/science/article/pii/S0168822708004646|journal=Diabetes Research and Clinical Practice|series=The Shiga International Symposium on Diabetic Nephropathy|language=en|volume=82|pages=S75–S79|doi=10.1016/j.diabres.2008.09.042|pmid=18994672|issn=0168-8227}}</ref> AGEs are chemical groups that form when a reducing sugar (glucose in this case) reacts non-enzymatically with an amine group, predominantly lysine and arginine, which are attached on proteins, lipids and nucleic acids.<ref>{{Cite journal|last1=Wolffenbuttel|first1=B. H. R.|last2=Boulanger|first2=C. M.|last3=Crijns|first3=F. R. L.|last4=Huijberts|first4=M. S. P.|last5=Poitevin|first5=P.|last6=Swennen|first6=G. N. M.|last7=Vasan|first7=S.|last8=Egan|first8=J. J.|last9=Ulrich|first9=P.|last10=Cerami|first10=A.|last11=Levy|first11=B. I.|date=1998-04-14|title=Breakers of advanced glycation end products restore large artery properties in experimental diabetes|journal=Proceedings of the National Academy of Sciences|volume=95|issue=8|pages=4630–4634|doi=10.1073/pnas.95.8.4630|pmid=9539789|pmc=22541|bibcode=1998PNAS...95.4630W|issn=0027-8424|doi-access=free}}</ref> These ] products accumulate on the proteins of vessel wall collagen, forming an irreversible complex of cross-linked AGEs. An important way AGEs exert their effect is through a receptor-mediated mechanism, most importantly by the receptor for advanced glycation end products (RAGE). RAGE is a signal transduction receptor found on a number of cell types including macrophages, endothelial cells, renal mesangial cells and podocytes in the glomerulus.<ref>{{Cite journal|last1=Yan|first1=Shi-Fang|last2=Ramasamy|first2=Ravichandran|last3=Bucciarelli|first3=Loredana G|last4=Wendt|first4=Thoralf|last5=Lee|first5=Larisse K|last6=Hudson|first6=Barry I|last7=Stenr|first7=David M|last8=Lalla|first8=Evanthia|last9=Du Yan|first9=Shi|last10=Rong|first10=Ling Ling|last11=Naka|first11=Yoshifumi|date=May 2004|title=RAGE and its ligands: a lasting memory in diabetic complications?|journal=Diabetes and Vascular Disease Research|volume=1|issue=1|pages=10–20|doi=10.3132/dvdr.2004.001|pmid=16305050|issn=1479-1641|doi-access=free}}</ref> Bindings of AGEs to RAGE receptors enhances production of cytosolic Reactive Oxygen Species (ROS) as well as stimulates intracellular molecules such as Protein Kinase C (PKC), NF-κB and the activation of growth factors TGF-B and vascular endothelial growth factor (VEGF). These factors, along with the hemodynamic changes that occur, lead to podocyte injury, oxidative stress, inflammation and fibrosis. As injury worsens, kidney function decreases and glomerular basement membrane (GBM) become more permeable and less efficient at filtration. This is accompanied by a steady decline in kidney function.{{citation needed|date=May 2020}} | |||
| Blood-glucose levels should be closely monitored and controlled. This may slow the progression of the disorder, especially in the very early ("microalbuminuria") stages. Medications to manage diabetes include oral hypoglycemic agents and ] injections. As kidney failure progresses, less insulin is excreted, so smaller doses may be needed to control glucose levels. | |||
| ==Diagnosis== | |||
| The ] may be modified to help control blood-sugar levels. | |||
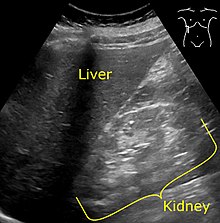
| ] showing ] of the ], visualized in the image as brighter than the liver.]] | |||
| Diagnosis is based on the measurement of abnormal levels of urinary albumin in an individual with diabetes <ref name="lewis20142">{{cite journal|vauthors=Lewis G, Maxwell AP|date=February 2014|title=Risk factor control is key in diabetic nephropathy|journal=The Practitioner|volume=258|issue=1768|pages=13–7, 2|pmid=24689163}}</ref> coupled with exclusion of other causes of albuminuria. Albumin measurements are defined as follows:<ref>{{Cite web|title=CDC – Chronic Kidney Disease – Glossary|url=http://nccd.cdc.gov/ckd/help.aspx?section=G|access-date=2015-07-02}}</ref> | |||
| :::::::::::* Normal ]: urinary albumin excretion <30 mg/24h; | |||
| High blood pressure should be aggressively treated with antihypertensive medications, in order to reduce the risks of kidney, eye, and blood vessel damage in the body. Therefore, it is the most effective way of slowing damage from diabetic nephropathy. It is also very important to control lipid levels, maintain a healthy weight, and engage in regular physical activity. | |||
| :::::::::::* ]: urinary albumin excretion in the range of 30–299 mg/24h; | |||
| :::::::::::* ]: urinary albumin excretion ≥300 mg/24h | |||
| ::::::::::: Urinary albumin excretion can also be measured by urinary albumin/creatinine ratio in a spot urine sample, which is as accurate but more convenient than a 24-hour urine collection.<ref>{{Cite journal|last1=Umanath|first1=Kausik|last2=Lewis|first2=Julia B.|date=2018-06-01|title=Update on Diabetic Nephropathy: Core Curriculum 2018|journal=American Journal of Kidney Diseases|language=en|volume=71|issue=6|pages=884–895|doi=10.1053/j.ajkd.2017.10.026|pmid=29398179|issn=0272-6386|doi-access=free}}</ref> | |||
| It is recommended that individuals with diabetes have their albumin levels checked annually, beginning immediately after a diagnosis of type 2 diabetes and five years after a diagnosis of type 1 diabetes.<ref name="lewis20142" /><ref>{{cite journal|vauthors=Koroshi A|date=July 2007|title=Microalbuminuria, is it so important?|journal=Hippokratia|volume=11|issue=3|pages=105–7|pmc=2658722|pmid=19582202}}</ref> ] of the kidneys, generally by ], is recommended as part of a ] if there is suspicion of ], ], ]s or ].<ref name="Grossde Azevedo20042">{{cite journal|vauthors=Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T|date=January 2005|title=Diabetic nephropathy: diagnosis, prevention, and treatment|journal=Diabetes Care|volume=28|issue=1|pages=164–76|doi=10.2337/diacare.28.1.164|pmid=15616252|doi-access=free}}</ref> Conformation kidney biopsy should only be performed if non-diabetic kidney disease is suspected.{{citation needed|date=January 2021}} | |||
| Patients with diabetic nephropathy should avoid taking the following drugs: | |||
| Urine analysis in patients with diabetic kidney disease is often bland. In cases of severely increased microalbuminuria, hematuria might be present.<ref>{{Cite journal|last1=Jiang|first1=Shimin|last2=Wang|first2=Yining|last3=Zhang|first3=Zheng|last4=Dai|first4=Peilin|last5=Yang|first5=Yue|last6=Li|first6=Wenge|date=September 2018|title=Accuracy of hematuria for predicting non-diabetic renal disease in patients with diabetes and kidney disease: A systematic review and meta-analysis|journal=Diabetes Research and Clinical Practice|volume=143|pages=288–300|doi=10.1016/j.diabres.2018.07.027|pmid=30059756|s2cid=51880893|issn=0168-8227}}</ref> fat bodies might be present in patients who develop nephrotic-range proteinuria. | |||
| * Contrast agents containing ] | |||
| {|class="wikitable" style="float:right; margin-left:5px" | |||
| * Commonly used non-steroidal anti-inflammatory drugs (]s) like ] and ], or ] inhibitors like ], because they may injure the weakened kidney. | |||
| |+ Clinical staging<ref name="pmid224391552">{{cite book|url=https://www.ncbi.nlm.nih.gov/books/NBK84558/|title=Chronic Kidney Disease Stages 1–3: Screening, Monitoring, and Treatment |vauthors=Fink HA, Ishani A, Taylor BC, Greer NL, MacDonald R, Rossini D, Sadiq S, Lankireddy S, Kane RL, Wilt TJ|chapter=Introduction |date=January 2012|publisher=Agency for Healthcare Research and Quality (US)|pmid=22439155|display-authors=6}}</ref> | |||
| !CKD<br>Stage | |||
| !eGFR level<br><small>(mL/min/1.73 m<sup>2</sup>)</small> | |||
| |- | |||
| |Stage 1 | |||
| | style="text-align:center" |≥ 90 | |||
| |- | |||
| |Stage 2 | |||
| | style="text-align:center" |60–89 | |||
| |- | |||
| |Stage 3 | |||
| | style="text-align:center" |30–59 | |||
| |- | |||
| |Stage 4 | |||
| | style="text-align:center" |15–29 | |||
| |- | |||
| |Stage 5 | |||
| | style="text-align:center" |< 15 | |||
| |} | |||
| ===Staging=== | |||
| ] and other ] are common and can be treated with appropriate ]. | |||
| To clinically stage the degree of damage in this (and any) kidney disease, the serum creatinine is determined and used to calculate the estimated glomerular filtration rate (]). Normal eGFR is equal to or greater than 90ml/min/1.73 m<sup>2</sup>.<ref>{{Cite web|title=Glomerular filtration rate: MedlinePlus Medical Encyclopedia|url=https://www.nlm.nih.gov/medlineplus/ency/article/007305.htm|website=www.nlm.nih.gov|access-date=2015-07-02}}</ref> | |||
| On biopsy, the following classification has been suggested by Tervaert ''et al.'':<ref name="pmid28316995">{{cite journal |last1=Qi |first1=Chenyang |last2=Mao |first2=Xing |last3=Zhang |first3=Zhigang |last4=Wu |first4=Huijuan |title=Classification and Differential Diagnosis of Diabetic Nephropathy |journal=Journal of Diabetes Research |date=2017 |volume=2017 |pages=8637138 |doi=10.1155/2017/8637138 |pmid=28316995 |pmc=5337846 |issn=2314-6745|doi-access=free }} ] Text was copied from this source, which is available under a . | |||
| </ref> | |||
| {|class=wikitable | |||
| |+Histopathologic staging | |||
| ! Class !! Description and criteria | |||
| |- | |||
| | I || Mild or nonspecific changes on light microscopy and conformed GBM<br>thickening proven by electron microscopy: GBM > 395 nm (female), GBM > 430 nm (male). | |||
| |- | |||
| | IIa | |||
| | | |||
| * Mild mesangial expansion in >25% of the observed mesangium. | |||
| * Area of mesangial proliferation < area of capillary cavity. | |||
| |- | |||
| | IIb | |||
| | | |||
| * Severe mesangial expansion in >25% of the observed mesangium. | |||
| * Area of mesangial proliferation < area of capillary cavity. | |||
| |- | |||
| | III | |||
| | At least one convincing nodular sclerosis (Kimmelstiel-Wilson lesion). | |||
| |- | |||
| | IV | |||
| | Advanced diabetic glomerulosclerosis in >50% of glomeruli. | |||
| |} | |||
| === Biomarkers === | |||
| ] may be necessary once end-stage renal disease develops. At this stage, a | |||
| Although albuminuria is the most frequently used marker of DN, it has a limited sensitivity as many patients with diabetic nephropathy experience GFR loss and glomerulosclerosis without immediate elevation in albuminuria. Many novel markers are currently being studied that potentially detect diabetic nephropathy at earlier stages and identify progression risk. Cystatin C is a protein that is freely filtered in the glomeruli before it is reabsorbed and catabolized in the renal tubular cells. Its serum level is independent of muscle mass, making more accurate at estimating GFR than creatinine serum levels.{{citation needed|date=May 2020}} | |||
| ] must be considered. Another option for type 1 diabetes patients is a combined kidney-pancreas transplant. | |||
| == Treatment == | |||
| The goals of treatment are to slow the progression of kidney damage and control related complications. Management of diabetic nephropathy currently centers over four main areas: Cardiovascular risk reduction, glycemic control, blood pressure control as well as inhibition of the RAAS system.{{citation needed|date=May 2020}} | |||
| Cardiovascular risk reduction: Patients with diabetes mellitus are at significantly increased risk of cardiovascular disease, which is also an independent risk factor for kidney failure. Therefore, it is important to aggressively manage cardiovascular risk factors in patients with diabetes mellitus and in particular those with diabetic nephropathy. The main components of managing cardiovascular disease is with tobacco cessation, lipid-lowering therapies (e.g., statins) as well as regular exercise and healthy eating.<ref>{{Cite journal|date=2008-06-12|title=Effects of Intensive Glucose Lowering in Type 2 Diabetes|journal=New England Journal of Medicine|volume=358|issue=24|pages=2545–2559|doi=10.1056/nejmoa0802743|pmid=18539917|pmc=4551392|issn=0028-4793|author1=Action to Control Cardiovascular Risk in Diabetes Study Group|last2=Gerstein|first2=H. C.|last3=Miller|first3=M. E.|last4=Byington|first4=R. P.|last5=Goff Jr|first5=D. C.|last6=Bigger|first6=J. T.|last7=Buse|first7=J. B.|last8=Cushman|first8=W. C.|last9=Genuth|first9=S.|last10=Ismail-Beigi|first10=F.|last11=Grimm Jr|first11=R. H.|last12=Probstfield|first12=J. L.|last13=Simons-Morton|first13=D. G.|last14=Friedewald|first14=W. T.}}</ref> In patients with kidney disease, atorvastatin is preferred over other statins as it does not require dose-adjustment based on GFR.<ref>{{Cite journal|last1=Bianchi|first1=Stefano|last2=Bigazzi|first2=Roberto|last3=Caiazza|first3=Alberto|last4=Campese|first4=Vito M.|date=2003-03-01|title=A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease|url=http://www.sciencedirect.com/science/article/pii/S0272638602692479|journal=American Journal of Kidney Diseases|language=en|volume=41|issue=3|pages=565–570|doi=10.1053/ajkd.2003.50140|pmid=12612979|issn=0272-6386}}</ref> | |||
| Glycemic control: Multiple studies have found a positive effect of improved glycemic control on clinical outcomes of patients with diabetic nephropathy.<ref>{{Cite journal|last1=DCCT/EDIC Research Group|last2=de Boer|first2=Ian H.|last3=Sun|first3=Wanjie|last4=Cleary|first4=Patricia A.|last5=Lachin|first5=John M.|last6=Molitch|first6=Mark E.|last7=Steffes|first7=Michael W.|last8=Zinman|first8=Bernard|date=2011-12-22|title=Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes|journal=The New England Journal of Medicine|volume=365|issue=25|pages=2366–2376|doi=10.1056/NEJMoa1111732|issn=1533-4406|pmc=3270008|pmid=22077236}}</ref> Intensive glycemic control also reduces the rate of other DM complications, such as retinopathy and neuropathy. Glycemic control is maintained mainly with insulin in patients with Type 1 DM and with hypoglycemic agents and/or insulin in patients with type 2 DM. Studies showed a decrease in microvascular complications of diabetic nephropathy with a target goal HbA1c concentration of 7%. Further reduction in the HbA1c did not correlate with better outcomes and is thus not recommended in most patients as it could increase the risk of hypoglycemic episodes.<ref>{{Cite journal|date=2008-06-12|title=Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes|journal=New England Journal of Medicine|volume=358|issue=24|pages=2560–2572|doi=10.1056/nejmoa0802987|pmid=18539916|issn=0028-4793|author1=ADVANCE Collaborative Group|last2=Patel|first2=A.|last3=MacMahon|first3=S.|last4=Chalmers|first4=J.|last5=Neal|first5=B.|last6=Billot|first6=L.|last7=Woodward|first7=M.|last8=Marre|first8=M.|last9=Cooper|first9=M.|last10=Glasziou|first10=P.|last11=Grobbee|first11=D.|last12=Hamet|first12=P.|last13=Harrap|first13=S.|last14=Heller|first14=S.|last15=Liu|first15=L.|last16=Mancia|first16=G.|last17=Mogensen|first17=C. E.|last18=Pan|first18=C.|last19=Poulter|first19=N.|last20=Rodgers|first20=A.|last21=Williams|first21=B.|last22=Bompoint|first22=S.|last23=De Galan|first23=B. E.|last24=Joshi|first24=R.|last25=Travert|first25=F.|url=https://espace.library.uq.edu.au/view/UQ:690128/UQ690128_OA.pdf|hdl=10072/26242|hdl-access=free}}</ref><ref>{{Cite journal|last1=Duckworth|first1=W.|last2=Abraira|first2=C.|last3=Moritz|first3=T.|date=April 2009|title=Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes|journal=Journal of Vascular Surgery|volume=49|issue=4|pages=129–39|doi=10.1016/j.jvs.2009.02.026|pmid=19092145|issn=0741-5214|doi-access=free}}</ref> | |||
| Blood pressure control: Multiple randomized clinical trials have demonstrated a benefit of decreasing systolic blood pressure to <140 mmHg in patients with diabetic nephropathy. High blood pressure is associated with accelerated development of microalbuminuria, over proteinuria and declining kidney function. Angiotensin-converting-enzyme inhibitors, as well as angiotensin II receptor blockers, are particularly helpful in patients with diabetes to lower blood pressure and slow the progression of nephropathy.<ref>{{Cite journal|date=2010-04-29|title=Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus|journal=New England Journal of Medicine|volume=362|issue=17|pages=1575–1585|doi=10.1056/nejmoa1001286|pmid=20228401|issn=0028-4793|author1=ACCORD Study Group|last2=Cushman|first2=W. C.|last3=Evans|first3=G. W.|last4=Byington|first4=R. P.|last5=Goff Jr|first5=D. C.|last6=Grimm Jr|first6=R. H.|last7=Cutler|first7=J. A.|last8=Simons-Morton|first8=D. G.|last9=Basile|first9=J. N.|last10=Corson|first10=M. A.|last11=Probstfield|first11=J. L.|last12=Katz|first12=L.|last13=Peterson|first13=K. A.|last14=Friedewald|first14=W. T.|last15=Buse|first15=J. B.|last16=Bigger|first16=J. T.|last17=Gerstein|first17=H. C.|last18=Ismail-Beigi|first18=F.|pmc=4123215}}</ref> More intensive blood pressure lower (125-130/<80) in patients with diabetic mellitus has been shown to decrease the risk of progression of diabetic nephropathy as well as other diabetic complications.<ref>{{Cite journal|title=Faculty Opinions recommendation of BP Control and Long-Term Risk of ESRD and Mortality.|last=Ruilope|first=Luis|date=2017-04-19|doi=10.3410/f.726630344.793530925 |doi-access=free }}</ref> Some patients might require dual therapy to adequately control pressure, in which case calcium channel blockers or diuretics are a good second-line option.<ref>{{Cite journal|title=Faculty Opinions recommendation of Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients.|last=Townsend|first=Raymond|date=2009-01-07|doi=10.3410/f.1142854.599941 |doi-access=free }}</ref> | |||
| RAAS inhibition: Inhibition can be achieved with multiple therapies, mainly ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors, and mineralocorticoid antagonists. RAAS inhibition has been proven to be the most effective therapy to slow the progression of diabetic nephropathy in all stages.<ref>{{Cite journal|last1=Lewis|first1=Edmund J.|last2=Hunsicker|first2=Lawrence G.|last3=Bain|first3=Raymond P.|last4=Rohde|first4=Richard D|date=1993-11-11|title=The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy|journal=New England Journal of Medicine|volume=329|issue=20|pages=1456–1462|doi=10.1056/nejm199311113292004|pmid=8413456|issn=0028-4793|doi-access=free}}</ref> Although RAAS blockade using more than one agent may further reduce proteinuria, the risk of adverse events (such as hyperkalemia, acute kidney injury) outweigh the potential benefits.<ref>{{Cite journal|last1=Fried|first1=Linda F.|last2=Emanuele|first2=Nicholas|last3=Zhang|first3=Jane H.|last4=Brophy|first4=Mary|last5=Conner|first5=Todd A.|last6=Duckworth|first6=William|last7=Leehey|first7=David J.|last8=McCullough|first8=Peter A.|last9=O'Connor|first9=Theresa|last10=Palevsky|first10=Paul M.|last11=Reilly|first11=Robert F.|date=2013-11-14|title=Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy|journal=New England Journal of Medicine|volume=369|issue=20|pages=1892–1903|doi=10.1056/nejmoa1303154|pmid=24206457|s2cid=205095632 |issn=0028-4793|doi-access=free}}</ref> Therefore, it is recommended that only one agent is used in patients with DM who have hypertension or any signs of microalbuminuria or diabetic nephropathy.<ref>{{Cite journal|last1=Brenner|first1=Barry M.|last2=Cooper|first2=Mark E.|last3=de Zeeuw|first3=Dick|last4=Keane|first4=William F.|last5=Mitch|first5=William E.|last6=Parving|first6=Hans-Henrik|last7=Remuzzi|first7=Giuseppe|last8=Snapinn|first8=Steven M.|last9=Zhang|first9=Zhonxin|last10=Shahinfar|first10=Shahnaz|date=2001-09-20|title=Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy|journal=New England Journal of Medicine|volume=345|issue=12|pages=861–869|doi=10.1056/nejmoa011161|pmid=11565518|hdl=2445/122643|issn=0028-4793|hdl-access=free}}</ref> | |||
| About half of insulin is metabolized and cleared by the kidneys. This means that as kidney function worsens in the setting of DN, some patients with insulin-dependent DM may find that their regular insulin doses are lasting longer than normal, or that they are experiencing an increasing frequency of hypoglycemic episodes. It is also crucial to closely monitor kidney function to properly dose medications that are cleared by the kidneys. Some of the most commonly used nephrotoxic medications are non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen.<ref>{{Cite journal|last1=Khan|first1=Kanwar Nasir M.|last2=Burke|first2=Allen|last3=Stanfield|first3=Kristina M.|last4=Harris|first4=Richard K.|last5=Baron|first5=David A.|date=2001-01-01|title=Expression of Cyclooxygenase-2 in the Macula Densa of Human Kidney in Hypertension, Congestive Heart Failure, and Diabetic Nephropathy|journal=Renal Failure|volume=23|issue=3–4|pages=321–330|doi=10.1081/JDI-100104716|pmid=11499548|s2cid=11727215|issn=0886-022X|doi-access=free}}</ref> | |||
| With worsening kidney function, it might also be necessary to follow a renal-diet to avoid complications such as hyperkalemia and metabolic acidosis. Some evidence suggests that limiting dietary protein could slow the progression of DN, but further evidence is needed to confirm this benefit.<ref>{{Cite journal|last1=Hansen|first1=Henrik P.|last2=Tauber-Lassen|first2=Ellis|last3=Jensen|first3=Berit R.|last4=Parving|first4=Hans-Henrik|date=July 2002|title=Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy|journal=Kidney International|volume=62|issue=1|pages=220–228|doi=10.1046/j.1523-1755.2002.00421.x|pmid=12081581|issn=0085-2538|doi-access=free}}</ref> Patients with diabetic nephropathy might go on to develop end stage renal disease and require kidney transplantation or hemodialysis.{{citation needed|date=May 2020}} | |||
| === Emerging therapies === | |||
| A relatively new medication that has been approved for treatment for DM is sodium glucose cotransporter 2 (SGLT2) inhibitors. The mechanism of action of this drug is to the sodium-glucose uptake cotransporter in the proximal tubule, thereby generating natriuresis and glucosuria. In multiple clinical trials, SGLT2 inhibitors showed improved cardiovascular outcomes in patients with DM as well a positive effect on kidney outcomes, mainly a reduction in albuminuria and progression of renal damage.<ref>{{Cite journal|last1=Heerspink|first1=Hiddo J.L.|last2=Perkins|first2=Bruce A.|last3=Fitchett|first3=David H.|last4=Husain|first4=Mansoor|last5=Cherney|first5=David Z. I.|date=2016-09-06|title=Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus|journal=Circulation|volume=134|issue=10|pages=752–772|doi=10.1161/circulationaha.116.021887|pmid=27470878|issn=0009-7322|doi-access=free}}</ref><ref>{{Cite journal|last1=Wanner|first1=Christoph|last2=Inzucchi|first2=Silvio E.|last3=Lachin|first3=John M.|last4=Fitchett|first4=David|last5=von Eynatten|first5=Maximilian|last6=Mattheus|first6=Michaela|last7=Johansen|first7=Odd Erik|last8=Woerle|first8=Hans J.|last9=Broedl|first9=Uli C.|last10=Zinman|first10=Bernard|date=2016-07-28|title=Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes|journal=New England Journal of Medicine|volume=375|issue=4|pages=323–334|doi=10.1056/nejmoa1515920|pmid=27299675|issn=0028-4793|doi-access=free}}</ref> Other classes of diabetic medications that have been shown to have a positive effect on the progression of diabetic nephropathy are GLP-1 agonists and DPP-4 inhibitors.{{citation needed|date=May 2020}} | |||
| === Education and self-management === | |||
| The success of diabetic nephropathy management depends greatly upon the ability of individuals to self-manage this condition, encompassing glycaemic control, and the adoption of healthy lifestyles. Appropriate self-management often requires patient education and behavioural counselling. However, there is still insufficient evidence to draw conclusions regarding the effects, regarding both benefits and harms, of educational programmes for people with diabetic nephropathy.<ref>{{Cite journal|last1=Li|first1=Ting|last2=Wu|first2=Hong Mei|last3=Wang|first3=Feng|last4=Huang|first4=Chang Quan|last5=Yang|first5=Ming|last6=Dong|first6=Bi Rong|last7=Liu|first7=Guan J|date=2011-06-15|editor-last=Cochrane Kidney and Transplant Group|title=Education programmes for people with diabetic kidney disease|url=https://doi.wiley.com/10.1002/14651858.CD007374.pub2|journal=Cochrane Database of Systematic Reviews|issue=6|pages=CD007374|language=en|doi=10.1002/14651858.CD007374.pub2|pmid=21678365}}</ref> | |||
| ==Prognosis== | ==Prognosis== | ||
| Diabetic nephropathy in type 2 diabetes can be more difficult to predict because the onset of diabetes is not usually well established. Without intervention, 20–40 percent of patients with type 2 diabetes/microalbuminuria, will evolve to macroalbuminuria.<ref>{{Cite journal|last=Shlipak|first=Michael|date=2011-03-15|title=Clinical Evidence Handbook: Diabetic Nephropathy: Preventing Progression – American Family Physician|url=http://www.aafp.org/afp/2011/0315/p732.html|journal=American Family Physician|volume=83|issue=6|pages=732|access-date=2015-06-27|name-list-style=vanc}}</ref> Diabetic nephropathy is the most common cause of ],<ref name="Fernandez201422"/><ref name="Ding201522"/> which may require ] or even ].<ref name="ReferenceB22"/> It is associated with an increased ] in general, particularly from ].<ref name="Fernandez201422" /><ref name="Pálsson R 201422"/> | |||
| == Epidemiology == | |||
| Diabetic nephropathy continues to get gradually worse. Complications of chronic kidney failure are more likely to occur earlier, and progress more rapidly, when it is caused by diabetes than other causes. Even after initiation of dialysis or after transplantation, people with diabetes tend to do worse than those without diabetes. | |||
| Diabetic nephropathy affects approximately a third of patients with type 1 and type 2 diabetes mellitus. Diabetic nephropathy is responsible for about a third of cases of ESRD worldwide, and an even larger fraction in the developed countries.<ref>{{Cite journal|last1=Zimmet|first1=Paul|last2=Alberti|first2=K. G. M. M.|last3=Shaw|first3=Jonathan|date=December 2001|title=Global and societal implications of the diabetes epidemic|journal=Nature|volume=414|issue=6865|pages=782–787|doi=10.1038/414782a|pmid=11742409|bibcode=2001Natur.414..782Z|s2cid=4384190|issn=0028-0836|url=https://repositorio.unal.edu.co/handle/unal/79579}}</ref> Worldwide, the prevalence of diabetes is projected to increase from 382 million in 2013, to over 592 million by 2035. This increase is projected to be sharpest in developed countries. The prevalence of type 2 DM is particularly increasing due to the rising prevalence of obesity worldwide.<ref>{{Cite journal|last1=Cameron|first1=Adrian J|last2=Zimmet|first2=Paul Z|last3=Atkins|first3=Robert C|last4=Shaw|first4=Jonathan E|date=2007|title=The Australian Diabetes, Obesity and Lifestyle Study – Profiling Diabetes and Cardiovascular Disease Risk in the Nation|journal=European Endocrinology|issue=2|pages=20|doi=10.17925/ee.2007.00.02.20|issn=1758-3772|doi-access=free}}</ref> Diabetic kidney disease progression could lead to ESRD as well as an increased risk of cardiovascular complications, all of which cause a substantial economic burden. The estimated cost of management of patients with ESRD due to diabetic nephropathy in the US is US$39.35 billion in 2010.<ref>{{Cite journal|last1=Trivedi|first1=Hariprasad S.|last2=Pang|first2=Michael M.H.|last3=Campbell|first3=Anne|last4=Saab|first4=Paulette|date=April 2002|title=Slowing the progression of chronic renal failure: Economic benefits and patients' perspectives|journal=American Journal of Kidney Diseases|volume=39|issue=4|pages=721–13|doi=10.1053/ajkd.2002.31990|pmid=11920337|issn=0272-6386}}</ref> Within developed countries, certain ethnic groups such as African Americans and Native Americans are at higher risk of developing diabetic nephropathy and ESRD.<ref>{{Cite journal|last=Disease|first=Ethnicity &|date=2018-10-17|title=Correction: Ethn Dis. 2010;20::S1-60-S1-64|journal=Ethnicity & Disease|volume=28|issue=4|pages=586|doi=10.18865/ed.28.4.586|pmid=30405305|pmc=6200305|issn=1945-0826|doi-access=free}}</ref> | |||
| == |
== See also == | ||
| * ] | |||
| Possible complications include: | |||
| * ] | |||
| * ] | |||
| == References == | |||
| * ] (from decreased excretion of insulin) | |||
| {{Reflist|32em}} | |||
| * rapidly progressing chronic ] | |||
| * ] | |||
| * ] | |||
| * severe ] | |||
| * complications of ] | |||
| * complications of ] | |||
| * coexistence of other ] complications | |||
| * ] (if peritoneal dialysis used) | |||
| * increased ] | |||
| == Further reading == | |||
| ==Reference== | |||
| {{refbegin|32em}} | |||
| * Kimmelstiel P, Wilson C. ''Benign and malignant hypertension and nephrosclerosis. A clinical and pathological study.'' Am J Pathol 1936;12:45-48. | |||
| * {{Cite web|title = Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: an updated meta-analysis|url = http://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=12009100199|website = www.crd.york.ac.uk|access-date = 2015-07-02}} | |||
| * {{cite journal | vauthors = Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T | title = Diabetic nephropathy: diagnosis, prevention, and treatment | journal = Diabetes Care | volume = 28 | issue = 1 | pages = 164–76 | date = January 2005 | pmid = 15616252 | doi = 10.2337/diacare.28.1.164 | doi-access = free }} | |||
| * {{cite journal | vauthors = Tziomalos K, Athyros VG | title = Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis | journal = The Review of Diabetic Studies | volume = 12 | issue = 1–2 | pages = 110–8 | year = 2015 | pmid = 26676664 | pmc = 5397986 | doi = 10.1900/RDS.2015.12.110 }} | |||
| * {{cite journal | vauthors = Kume S, Koya D, Uzu T, Maegawa H | title = Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy | journal = BioMed Research International | volume = 2014 | pages = 315494 | date = 2014 | pmid = 25126552 | pmc = 4122096 | doi = 10.1155/2014/315494 | doi-access = free }} | |||
| * {{cite journal | vauthors = Doshi SM, Friedman AN | title = Diagnosis and Management of Type 2 Diabetic Kidney Disease | journal = Clinical Journal of the American Society of Nephrology | volume = 12 | issue = 8 | pages = 1366–1373 | date = August 2017 | pmid = 28280116 | pmc = 5544517 | doi = 10.2215/CJN.11111016 }} | |||
| {{refend}} | |||
| == External links == | |||
| {{Medical resources | |||
| | DiseasesDB = | |||
| | ICD10 = E10.2, E11.2, E12.2, E13.2, E14.2 | |||
| | ICD9 = {{ICD9|250.4}} | |||
| | ICDO = | |||
| | OMIM = | |||
| | MedlinePlus = 000494 | |||
| | eMedicineSubj = | |||
| | eMedicineTopic = | |||
| | MeshID = D003928 | |||
| }} | |||
| {{Scholia|topic}} | |||
| {{Glomerular disease}} | |||
| {{Diabetes}} | |||
| ==External links== | |||
| * . HealthCentral. | |||
| * . MedlinePlus Medical Encyclopedia. Text from this public domain article was partially used here. | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| {{DEFAULTSORT:Diabetic Nephropathy}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 16:24, 29 December 2024
Chronic loss of kidney function Medical condition| Diabetic nephropathy | |
|---|---|
| Other names | Diabetic kidney disease |
 | |
| Two glomeruli in diabetic nephropathy: the acellular light purple areas within the capillary tufts are the destructive mesangial matrix deposits. | |
| Specialty | Nephrology, endocrinology |
| Symptoms | Albuminuria, peripheral edema, high blood pressure, tiredness |
| Risk factors | High blood pressure, tobacco smoking, unstable blood glucose |
| Diagnostic method | Abnormal levels of urinary albumin |
| Prevention | Smoking cessation |
| Treatment | ACE inhibitors |
Diabetic nephropathy, also known as diabetic kidney disease, is the chronic loss of kidney function occurring in those with diabetes mellitus. Diabetic nephropathy is the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) globally. The triad of protein leaking into the urine (proteinuria or albuminuria), rising blood pressure with hypertension and then falling renal function is common to many forms of CKD. Protein loss in the urine due to damage of the glomeruli may become massive, and cause a low serum albumin with resulting generalized body swelling (edema) so called nephrotic syndrome. Likewise, the estimated glomerular filtration rate (eGFR) may progressively fall from a normal of over 90 ml/min/1.73m to less than 15, at which point the patient is said to have end-stage renal disease. It usually is slowly progressive over years.
Pathophysiologic abnormalities in diabetic nephropathy usually begin with long-standing poorly controlled blood glucose levels. This is followed by multiple changes in the filtration units of the kidneys, the nephrons. (There are normally about 750,000–1.5 million nephrons in each adult kidney). Initially, there is constriction of the efferent arterioles and dilation of afferent arterioles, with resulting glomerular capillary hypertension and hyperfiltration particularly as nephrons become obsolescent and the adaption of hyperfiltration paradoxically causes further shear stress related damage to the delicate glomerular capillaries, further proteinuria, rising blood pressure and a vicious circle of additional nephron damage and decline in overall renal function. Concurrently, there are changes within the glomerulus itself: these include a thickening of the basement membrane, a widening of the slit membranes of the podocytes, an increase in the number of mesangial cells, and an increase in mesangial matrix. This matrix invades the glomerular capillaries and produces deposits called Kimmelstiel-Wilson nodules. The mesangial cells and matrix can progressively expand and consume the entire glomerulus, shutting off filtration.
The status of diabetic nephropathy may be monitored by measuring two values: the amount of protein in the urine - proteinuria; and a blood test called the serum creatinine. The amount of the proteinuria reflects the degree of damage to any still-functioning glomeruli. The value of the serum creatinine can be used to calculate the estimated glomerular filtration rate (eGFR), which reflects the percentage of glomeruli which are no longer filtering the blood. Treatment with an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, which dilates the arteriole exiting the glomerulus, thus reducing the blood pressure within the glomerular capillaries, may slow (but not stop) progression of the disease. Three classes of diabetes medications – GLP-1 agonists, DPP-4 inhibitors, and SGLT2 inhibitors– are also thought to slow the progression of diabetic nephropathy.
Diabetic nephropathy is the most common cause of end-stage renal disease and is a serious complication that affects approximately one quarter of adults with diabetes in the United States. Affected individuals with end-stage kidney disease often require hemodialysis and eventually kidney transplantation to replace the failed kidney function. Diabetic nephropathy is associated with an increased risk of death in general, particularly from cardiovascular disease.
Signs and symptoms
The onset of symptoms is 5 to 10 years after the disease begins. A usual first symptom is frequent urination at night: nocturia. Other symptoms include tiredness, headaches, a general feeling of illness, nausea, vomiting, frequent daytime urination, lack of appetite, itchy skin, and leg swelling. The clinical presentation of diabetic nephropathy (DN) is characterized by proteinuria (protein in the urine), hypertension and progressive loss of kidney function. The process may be initially indolent, making regular screening for diabetic nephropathy in patients with diabetes mellitus of great importance.
Risk factors
Not all patients with diabetes go on to develop diabetic nephropathy. The main risk factors that increase the likelihood of developing diabetic nephropathy are:
- Poor control of blood glucose
- Uncontrolled high blood pressure
- Type 1 diabetes mellitus, with onset before age 20
- Past or current cigarette use
- A family history of diabetic nephropathy- certain genes have been identified that are associated with DN. ( However, no direct correlation has been established yet. One of these genes is APOL1, which has been found to be associated with nephropathy in African American individuals.)
- Certain racial groups (African Americans, Mexican Americans, and Pima Indians are at higher risk).
Pathophysiology

The disease progression of diabetic nephropathy involves various clinical stages: hyperfiltration, microalbuminuria, macroalbuminuria, nephrotic proteinuria to progressive chronic kidney disease leading to end-stage renal disease (ESRD). The damage is exerted on all compartments of the kidney: the glomerulus, the renal tubules, the vasculature (afferent and efferent renal arterioles) and the interstitium. Renal fibrosis is the final common pathway of DN. This fibrosis is a product of multiple mechanisms including renal hemodynamic changes, glucose metabolism abnormalities associated with oxidative stress as well as inflammatory processes and an overactive renin-angiotensin-aldosterone system (RAAS).
The pathophysiology of diabetic nephropathy is thought to involve an interaction between hemodynamic and metabolic factors.
Hemodynamic factors include an increase in systemic and intraglomerular pressure, as well as the over-activation of the RAAS. Studies have shown that in the setting of diabetes, various factors stimulate the RAAS, which is one of the most important pathways in diabetic nephropathy pathophysiology. Due to the higher load of filtered glucose, there is an up-regulation in the sodium-glucose cotransporter 2 (SGLT2) in the proximal tubules, which cotransports sodium and glucose back into circulation. This leads to a decrease in the delivery of sodium chloride to the macula densa in the distal tubules, promoting the release of renin and over-activating RAAS. Hyperfiltration is one of the earliest features of DN. Several mechanisms have been proposed to cause hyperfiltration. One of these mechanisms is that as glomeruli becomes hypertrophied, filtration surface area initially increases. Another possible mechanism is that abnormal vascular control in diabetic nephropathy leads to a reduction in afferent glomerular arteriolar resistance and an increase in efferent glomerular arteriolar resistance, leading to a net increase in renal blood flow (RBF) and glomerular filtration rate (GFR). Glomerular hyperfiltration and an aberrant regulation of RAAS lead to increased intraglomerular pressure, causing stress on the endothelial cells, the mesangial cells and the podocytes. This exacerbates the dysfunction caused by the metabolic effects of hyperglycemia.
Metabolic factors include the formation of advanced glycation end-products (AGEs), which have a central role in the pathophysiology of many of the complications of diabetes mellitus, including cardiovascular complications. AGEs are chemical groups that form when a reducing sugar (glucose in this case) reacts non-enzymatically with an amine group, predominantly lysine and arginine, which are attached on proteins, lipids and nucleic acids. These glycation products accumulate on the proteins of vessel wall collagen, forming an irreversible complex of cross-linked AGEs. An important way AGEs exert their effect is through a receptor-mediated mechanism, most importantly by the receptor for advanced glycation end products (RAGE). RAGE is a signal transduction receptor found on a number of cell types including macrophages, endothelial cells, renal mesangial cells and podocytes in the glomerulus. Bindings of AGEs to RAGE receptors enhances production of cytosolic Reactive Oxygen Species (ROS) as well as stimulates intracellular molecules such as Protein Kinase C (PKC), NF-κB and the activation of growth factors TGF-B and vascular endothelial growth factor (VEGF). These factors, along with the hemodynamic changes that occur, lead to podocyte injury, oxidative stress, inflammation and fibrosis. As injury worsens, kidney function decreases and glomerular basement membrane (GBM) become more permeable and less efficient at filtration. This is accompanied by a steady decline in kidney function.
Diagnosis
Diagnosis is based on the measurement of abnormal levels of urinary albumin in an individual with diabetes coupled with exclusion of other causes of albuminuria. Albumin measurements are defined as follows:
- Normal albuminuria: urinary albumin excretion <30 mg/24h;
- Microalbuminuria: urinary albumin excretion in the range of 30–299 mg/24h;
- Macroalbuminuria: urinary albumin excretion ≥300 mg/24h
- Urinary albumin excretion can also be measured by urinary albumin/creatinine ratio in a spot urine sample, which is as accurate but more convenient than a 24-hour urine collection.
It is recommended that individuals with diabetes have their albumin levels checked annually, beginning immediately after a diagnosis of type 2 diabetes and five years after a diagnosis of type 1 diabetes. Medical imaging of the kidneys, generally by ultrasonography, is recommended as part of a differential diagnosis if there is suspicion of urinary tract obstruction, urinary tract infection, kidney stones or polycystic kidney disease. Conformation kidney biopsy should only be performed if non-diabetic kidney disease is suspected.
Urine analysis in patients with diabetic kidney disease is often bland. In cases of severely increased microalbuminuria, hematuria might be present. fat bodies might be present in patients who develop nephrotic-range proteinuria.
| CKD Stage |
eGFR level (mL/min/1.73 m) |
|---|---|
| Stage 1 | ≥ 90 |
| Stage 2 | 60–89 |
| Stage 3 | 30–59 |
| Stage 4 | 15–29 |
| Stage 5 | < 15 |
Staging
To clinically stage the degree of damage in this (and any) kidney disease, the serum creatinine is determined and used to calculate the estimated glomerular filtration rate (eGFR). Normal eGFR is equal to or greater than 90ml/min/1.73 m. On biopsy, the following classification has been suggested by Tervaert et al.:
| Class | Description and criteria |
|---|---|
| I | Mild or nonspecific changes on light microscopy and conformed GBM thickening proven by electron microscopy: GBM > 395 nm (female), GBM > 430 nm (male). |
| IIa |
|
| IIb |
|
| III | At least one convincing nodular sclerosis (Kimmelstiel-Wilson lesion). |
| IV | Advanced diabetic glomerulosclerosis in >50% of glomeruli. |
Biomarkers
Although albuminuria is the most frequently used marker of DN, it has a limited sensitivity as many patients with diabetic nephropathy experience GFR loss and glomerulosclerosis without immediate elevation in albuminuria. Many novel markers are currently being studied that potentially detect diabetic nephropathy at earlier stages and identify progression risk. Cystatin C is a protein that is freely filtered in the glomeruli before it is reabsorbed and catabolized in the renal tubular cells. Its serum level is independent of muscle mass, making more accurate at estimating GFR than creatinine serum levels.
Treatment
The goals of treatment are to slow the progression of kidney damage and control related complications. Management of diabetic nephropathy currently centers over four main areas: Cardiovascular risk reduction, glycemic control, blood pressure control as well as inhibition of the RAAS system.
Cardiovascular risk reduction: Patients with diabetes mellitus are at significantly increased risk of cardiovascular disease, which is also an independent risk factor for kidney failure. Therefore, it is important to aggressively manage cardiovascular risk factors in patients with diabetes mellitus and in particular those with diabetic nephropathy. The main components of managing cardiovascular disease is with tobacco cessation, lipid-lowering therapies (e.g., statins) as well as regular exercise and healthy eating. In patients with kidney disease, atorvastatin is preferred over other statins as it does not require dose-adjustment based on GFR.
Glycemic control: Multiple studies have found a positive effect of improved glycemic control on clinical outcomes of patients with diabetic nephropathy. Intensive glycemic control also reduces the rate of other DM complications, such as retinopathy and neuropathy. Glycemic control is maintained mainly with insulin in patients with Type 1 DM and with hypoglycemic agents and/or insulin in patients with type 2 DM. Studies showed a decrease in microvascular complications of diabetic nephropathy with a target goal HbA1c concentration of 7%. Further reduction in the HbA1c did not correlate with better outcomes and is thus not recommended in most patients as it could increase the risk of hypoglycemic episodes.
Blood pressure control: Multiple randomized clinical trials have demonstrated a benefit of decreasing systolic blood pressure to <140 mmHg in patients with diabetic nephropathy. High blood pressure is associated with accelerated development of microalbuminuria, over proteinuria and declining kidney function. Angiotensin-converting-enzyme inhibitors, as well as angiotensin II receptor blockers, are particularly helpful in patients with diabetes to lower blood pressure and slow the progression of nephropathy. More intensive blood pressure lower (125-130/<80) in patients with diabetic mellitus has been shown to decrease the risk of progression of diabetic nephropathy as well as other diabetic complications. Some patients might require dual therapy to adequately control pressure, in which case calcium channel blockers or diuretics are a good second-line option.
RAAS inhibition: Inhibition can be achieved with multiple therapies, mainly ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors, and mineralocorticoid antagonists. RAAS inhibition has been proven to be the most effective therapy to slow the progression of diabetic nephropathy in all stages. Although RAAS blockade using more than one agent may further reduce proteinuria, the risk of adverse events (such as hyperkalemia, acute kidney injury) outweigh the potential benefits. Therefore, it is recommended that only one agent is used in patients with DM who have hypertension or any signs of microalbuminuria or diabetic nephropathy.
About half of insulin is metabolized and cleared by the kidneys. This means that as kidney function worsens in the setting of DN, some patients with insulin-dependent DM may find that their regular insulin doses are lasting longer than normal, or that they are experiencing an increasing frequency of hypoglycemic episodes. It is also crucial to closely monitor kidney function to properly dose medications that are cleared by the kidneys. Some of the most commonly used nephrotoxic medications are non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen. With worsening kidney function, it might also be necessary to follow a renal-diet to avoid complications such as hyperkalemia and metabolic acidosis. Some evidence suggests that limiting dietary protein could slow the progression of DN, but further evidence is needed to confirm this benefit. Patients with diabetic nephropathy might go on to develop end stage renal disease and require kidney transplantation or hemodialysis.
Emerging therapies
A relatively new medication that has been approved for treatment for DM is sodium glucose cotransporter 2 (SGLT2) inhibitors. The mechanism of action of this drug is to the sodium-glucose uptake cotransporter in the proximal tubule, thereby generating natriuresis and glucosuria. In multiple clinical trials, SGLT2 inhibitors showed improved cardiovascular outcomes in patients with DM as well a positive effect on kidney outcomes, mainly a reduction in albuminuria and progression of renal damage. Other classes of diabetic medications that have been shown to have a positive effect on the progression of diabetic nephropathy are GLP-1 agonists and DPP-4 inhibitors.
Education and self-management
The success of diabetic nephropathy management depends greatly upon the ability of individuals to self-manage this condition, encompassing glycaemic control, and the adoption of healthy lifestyles. Appropriate self-management often requires patient education and behavioural counselling. However, there is still insufficient evidence to draw conclusions regarding the effects, regarding both benefits and harms, of educational programmes for people with diabetic nephropathy.
Prognosis
Diabetic nephropathy in type 2 diabetes can be more difficult to predict because the onset of diabetes is not usually well established. Without intervention, 20–40 percent of patients with type 2 diabetes/microalbuminuria, will evolve to macroalbuminuria. Diabetic nephropathy is the most common cause of end-stage kidney disease, which may require hemodialysis or even kidney transplantation. It is associated with an increased risk of death in general, particularly from cardiovascular disease.
Epidemiology
Diabetic nephropathy affects approximately a third of patients with type 1 and type 2 diabetes mellitus. Diabetic nephropathy is responsible for about a third of cases of ESRD worldwide, and an even larger fraction in the developed countries. Worldwide, the prevalence of diabetes is projected to increase from 382 million in 2013, to over 592 million by 2035. This increase is projected to be sharpest in developed countries. The prevalence of type 2 DM is particularly increasing due to the rising prevalence of obesity worldwide. Diabetic kidney disease progression could lead to ESRD as well as an increased risk of cardiovascular complications, all of which cause a substantial economic burden. The estimated cost of management of patients with ESRD due to diabetic nephropathy in the US is US$39.35 billion in 2010. Within developed countries, certain ethnic groups such as African Americans and Native Americans are at higher risk of developing diabetic nephropathy and ESRD.
See also
References
- ^ Alamo, A.; Campagna, D.; Di Pino, A.; Russo, C.; Calogero, A. E.; Polosa, R.; Purrello, F. (October 2019). "Smoking and diabetes: dangerous liaisons and confusing relationships" (PDF). Diabetology & Metabolic Syndrome. 11 (85). BioMed Central: 85. doi:10.1186/s13098-019-0482-2. ISSN 1758-5996. PMC 6813988. PMID 31666811. S2CID 204882089. Retrieved 20 August 2021.
- ^ "Diabetes and kidney disease: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2015-06-27.
- Lewis G, Maxwell AP (February 2014). "Risk factor control is key in diabetic nephropathy". The Practitioner. 258 (1768): 13–7, 2. PMID 24689163.
- Lim AK (2014). "Diabetic nephropathy – complications and treatment". International Journal of Nephrology and Renovascular Disease. 7: 361–81. doi:10.2147/IJNRD.S40172. PMC 4206379. PMID 25342915.
- Kittell F (2012). "Diabetes Management". In Thomas LK, Othersen JB (eds.). Nutrition Therapy for Chronic Kidney Disease. CRC Press. p. 198. ISBN 9781439849491.
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (2013). Harrison's manual of medicine (18th ed.). New York: McGraw-Hill Medical. p. 2982. ISBN 978-0-07-174519-2.
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH (August 2016). "Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014". JAMA. 316 (6): 602–10. doi:10.1001/jama.2016.10924. PMC 5444809. PMID 27532915.
- Hall J, Guyton A (2005). Textbook of Medical Physiology (11th ed.). Philadelphia: W.B. Saunders. p. 310. ISBN 978-0-7216-0240-0.
- Hostetter, T. H.; Olson, J. L.; Rennke, H. G.; Venkatachalam, M. A.; Brenner, B. M. (July 1981). "Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation". The American Journal of Physiology. 241 (1): F85–93. doi:10.1152/ajprenal.1981.241.1.F85. ISSN 0002-9513. PMID 7246778. S2CID 1553863.
- "diabetic nephropathy". Retrieved 2015-06-27.
- Schlöndorff D, Banas B (June 2009). "The mesangial cell revisited: no cell is an island". Journal of the American Society of Nephrology. 20 (6): 1179–87. doi:10.1681/ASN.2008050549. PMID 19470685.
- de Boer IH (August 2017). "A New Chapter for Diabetic Kidney Disease". The New England Journal of Medicine. 377 (9): 885–887. doi:10.1056/nejme1708949. PMID 28854097.
- ^ Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF (September 2014). "Diabetic kidney disease: from physiology to therapeutics". The Journal of Physiology. 592 (18): 3997–4012. doi:10.1113/jphysiol.2014.272328. PMC 4198010. PMID 24907306.
- ^ Ding Y, Choi ME (January 2015). "Autophagy in diabetic nephropathy". The Journal of Endocrinology. 224 (1): R15–30. doi:10.1530/JOE-14-0437. PMC 4238413. PMID 25349246.
- ^ Lizicarova D, Krahulec B, Hirnerova E, Gaspar L, Celecova Z (2014). "Risk factors in diabetic nephropathy progression at present". Bratislavske Lekarske Listy. 115 (8): 517–21. doi:10.4149/BLL_2014_101. PMID 25246291.
- ^ Pálsson R, Patel UD (May 2014). "Cardiovascular complications of diabetic kidney disease". Advances in Chronic Kidney Disease. 21 (3): 273–80. doi:10.1053/j.ackd.2014.03.003. PMC 4045477. PMID 24780455.
- Kussman, M. J. (1976-10-18). "The clinical course of diabetic nephropathy". Journal of the American Medical Association. 236 (16): 1861–1863. doi:10.1001/jama.236.16.1861. ISSN 0098-7484. PMID 989537.
- Jiang N (Nov 2017). "Smoking and the risk of diabetic nephropathy in patients with type 1 and type 2 diabetes: a meta-analysis of observational studies". Oncotarget. 8 (54): 93209–93218. doi:10.18632/oncotarget.21478. PMC 5696256. PMID 29190990.
- Freedman, Barry I.; Bostrom, Meredith; Daeihagh, Pirouz; Bowden, Donald W. (2007-10-17). "Genetic Factors in Diabetic Nephropathy". Clinical Journal of the American Society of Nephrology. 2 (6): 1306–1316. doi:10.2215/cjn.02560607. ISSN 1555-9041. PMID 17942768.
- Kruzel-Davila, Etty; Wasser, Walter G.; Aviram, Sharon; Skorecki, Karl (March 2016). "APOL1 nephropathy: from gene to mechanisms of kidney injury". Nephrology, Dialysis, Transplantation. 31 (3): 349–358. doi:10.1093/ndt/gfu391. ISSN 1460-2385. PMID 25561578.
- Lin, Yi-Chih; Chang, Yu-Hsing; Yang, Shao-Yu; Wu, Kwan-Dun; Chu, Tzong-Shinn (2018-08-01). "Update of pathophysiology and management of diabetic kidney disease". Journal of the Formosan Medical Association. 117 (8): 662–675. doi:10.1016/j.jfma.2018.02.007. ISSN 0929-6646. PMID 29486908.
- Anderson, Sharon; Brenner, Barry M. (1988), "Pathogenesis of Diabetic Glomerulopathy: The Role of Glomerular Hyperfiltration", The Kidney and Hypertension in Diabetes Mellitus, Springer US, pp. 139–146, doi:10.1007/978-1-4757-1974-1_17, ISBN 978-1-4757-1976-5
- Hostetter, Thomas H. (March 2003). "Hyperfiltration and glomerulosclerosis". Seminars in Nephrology. 23 (2): 194–199. doi:10.1053/anep.2003.50017. ISSN 0270-9295. PMID 12704579.
- Soldatos, G.; Cooper, M. E. (2008-11-13). "Diabetic nephropathy: Important pathophysiologic mechanisms". Diabetes Research and Clinical Practice. The Shiga International Symposium on Diabetic Nephropathy. 82: S75 – S79. doi:10.1016/j.diabres.2008.09.042. ISSN 0168-8227. PMID 18994672.
- Wolffenbuttel, B. H. R.; Boulanger, C. M.; Crijns, F. R. L.; Huijberts, M. S. P.; Poitevin, P.; Swennen, G. N. M.; Vasan, S.; Egan, J. J.; Ulrich, P.; Cerami, A.; Levy, B. I. (1998-04-14). "Breakers of advanced glycation end products restore large artery properties in experimental diabetes". Proceedings of the National Academy of Sciences. 95 (8): 4630–4634. Bibcode:1998PNAS...95.4630W. doi:10.1073/pnas.95.8.4630. ISSN 0027-8424. PMC 22541. PMID 9539789.
- Yan, Shi-Fang; Ramasamy, Ravichandran; Bucciarelli, Loredana G; Wendt, Thoralf; Lee, Larisse K; Hudson, Barry I; Stenr, David M; Lalla, Evanthia; Du Yan, Shi; Rong, Ling Ling; Naka, Yoshifumi (May 2004). "RAGE and its ligands: a lasting memory in diabetic complications?". Diabetes and Vascular Disease Research. 1 (1): 10–20. doi:10.3132/dvdr.2004.001. ISSN 1479-1641. PMID 16305050.
- ^ Lewis G, Maxwell AP (February 2014). "Risk factor control is key in diabetic nephropathy". The Practitioner. 258 (1768): 13–7, 2. PMID 24689163.
- "CDC – Chronic Kidney Disease – Glossary". Retrieved 2015-07-02.
- Umanath, Kausik; Lewis, Julia B. (2018-06-01). "Update on Diabetic Nephropathy: Core Curriculum 2018". American Journal of Kidney Diseases. 71 (6): 884–895. doi:10.1053/j.ajkd.2017.10.026. ISSN 0272-6386. PMID 29398179.
- Koroshi A (July 2007). "Microalbuminuria, is it so important?". Hippokratia. 11 (3): 105–7. PMC 2658722. PMID 19582202.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (January 2005). "Diabetic nephropathy: diagnosis, prevention, and treatment". Diabetes Care. 28 (1): 164–76. doi:10.2337/diacare.28.1.164. PMID 15616252.
- Jiang, Shimin; Wang, Yining; Zhang, Zheng; Dai, Peilin; Yang, Yue; Li, Wenge (September 2018). "Accuracy of hematuria for predicting non-diabetic renal disease in patients with diabetes and kidney disease: A systematic review and meta-analysis". Diabetes Research and Clinical Practice. 143: 288–300. doi:10.1016/j.diabres.2018.07.027. ISSN 0168-8227. PMID 30059756. S2CID 51880893.
- Fink HA, Ishani A, Taylor BC, Greer NL, MacDonald R, Rossini D, et al. (January 2012). "Introduction". Chronic Kidney Disease Stages 1–3: Screening, Monitoring, and Treatment [Internet]. Agency for Healthcare Research and Quality (US). PMID 22439155.
- "Glomerular filtration rate: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2015-07-02.
- Qi, Chenyang; Mao, Xing; Zhang, Zhigang; Wu, Huijuan (2017). "Classification and Differential Diagnosis of Diabetic Nephropathy". Journal of Diabetes Research. 2017: 8637138. doi:10.1155/2017/8637138. ISSN 2314-6745. PMC 5337846. PMID 28316995.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H. C.; Miller, M. E.; Byington, R. P.; Goff Jr, D. C.; Bigger, J. T.; Buse, J. B.; Cushman, W. C.; Genuth, S.; Ismail-Beigi, F.; Grimm Jr, R. H.; Probstfield, J. L.; Simons-Morton, D. G.; Friedewald, W. T. (2008-06-12). "Effects of Intensive Glucose Lowering in Type 2 Diabetes". New England Journal of Medicine. 358 (24): 2545–2559. doi:10.1056/nejmoa0802743. ISSN 0028-4793. PMC 4551392. PMID 18539917.
- Bianchi, Stefano; Bigazzi, Roberto; Caiazza, Alberto; Campese, Vito M. (2003-03-01). "A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease". American Journal of Kidney Diseases. 41 (3): 565–570. doi:10.1053/ajkd.2003.50140. ISSN 0272-6386. PMID 12612979.
- DCCT/EDIC Research Group; de Boer, Ian H.; Sun, Wanjie; Cleary, Patricia A.; Lachin, John M.; Molitch, Mark E.; Steffes, Michael W.; Zinman, Bernard (2011-12-22). "Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes". The New England Journal of Medicine. 365 (25): 2366–2376. doi:10.1056/NEJMoa1111732. ISSN 1533-4406. PMC 3270008. PMID 22077236.
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; Hamet, P.; Harrap, S.; Heller, S.; Liu, L.; Mancia, G.; Mogensen, C. E.; Pan, C.; Poulter, N.; Rodgers, A.; Williams, B.; Bompoint, S.; De Galan, B. E.; Joshi, R.; Travert, F. (2008-06-12). "Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes" (PDF). New England Journal of Medicine. 358 (24): 2560–2572. doi:10.1056/nejmoa0802987. hdl:10072/26242. ISSN 0028-4793. PMID 18539916.
- Duckworth, W.; Abraira, C.; Moritz, T. (April 2009). "Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes". Journal of Vascular Surgery. 49 (4): 129–39. doi:10.1016/j.jvs.2009.02.026. ISSN 0741-5214. PMID 19092145.
- ACCORD Study Group; Cushman, W. C.; Evans, G. W.; Byington, R. P.; Goff Jr, D. C.; Grimm Jr, R. H.; Cutler, J. A.; Simons-Morton, D. G.; Basile, J. N.; Corson, M. A.; Probstfield, J. L.; Katz, L.; Peterson, K. A.; Friedewald, W. T.; Buse, J. B.; Bigger, J. T.; Gerstein, H. C.; Ismail-Beigi, F. (2010-04-29). "Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus". New England Journal of Medicine. 362 (17): 1575–1585. doi:10.1056/nejmoa1001286. ISSN 0028-4793. PMC 4123215. PMID 20228401.
- Ruilope, Luis (2017-04-19). "Faculty Opinions recommendation of BP Control and Long-Term Risk of ESRD and Mortality". doi:10.3410/f.726630344.793530925.
{{cite journal}}: Cite journal requires|journal=(help) - Townsend, Raymond (2009-01-07). "Faculty Opinions recommendation of Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients". doi:10.3410/f.1142854.599941.
{{cite journal}}: Cite journal requires|journal=(help) - Lewis, Edmund J.; Hunsicker, Lawrence G.; Bain, Raymond P.; Rohde, Richard D (1993-11-11). "The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy". New England Journal of Medicine. 329 (20): 1456–1462. doi:10.1056/nejm199311113292004. ISSN 0028-4793. PMID 8413456.
- Fried, Linda F.; Emanuele, Nicholas; Zhang, Jane H.; Brophy, Mary; Conner, Todd A.; Duckworth, William; Leehey, David J.; McCullough, Peter A.; O'Connor, Theresa; Palevsky, Paul M.; Reilly, Robert F. (2013-11-14). "Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy". New England Journal of Medicine. 369 (20): 1892–1903. doi:10.1056/nejmoa1303154. ISSN 0028-4793. PMID 24206457. S2CID 205095632.
- Brenner, Barry M.; Cooper, Mark E.; de Zeeuw, Dick; Keane, William F.; Mitch, William E.; Parving, Hans-Henrik; Remuzzi, Giuseppe; Snapinn, Steven M.; Zhang, Zhonxin; Shahinfar, Shahnaz (2001-09-20). "Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy". New England Journal of Medicine. 345 (12): 861–869. doi:10.1056/nejmoa011161. hdl:2445/122643. ISSN 0028-4793. PMID 11565518.
- Khan, Kanwar Nasir M.; Burke, Allen; Stanfield, Kristina M.; Harris, Richard K.; Baron, David A. (2001-01-01). "Expression of Cyclooxygenase-2 in the Macula Densa of Human Kidney in Hypertension, Congestive Heart Failure, and Diabetic Nephropathy". Renal Failure. 23 (3–4): 321–330. doi:10.1081/JDI-100104716. ISSN 0886-022X. PMID 11499548. S2CID 11727215.
- Hansen, Henrik P.; Tauber-Lassen, Ellis; Jensen, Berit R.; Parving, Hans-Henrik (July 2002). "Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy". Kidney International. 62 (1): 220–228. doi:10.1046/j.1523-1755.2002.00421.x. ISSN 0085-2538. PMID 12081581.
- Heerspink, Hiddo J.L.; Perkins, Bruce A.; Fitchett, David H.; Husain, Mansoor; Cherney, David Z. I. (2016-09-06). "Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus". Circulation. 134 (10): 752–772. doi:10.1161/circulationaha.116.021887. ISSN 0009-7322. PMID 27470878.
- Wanner, Christoph; Inzucchi, Silvio E.; Lachin, John M.; Fitchett, David; von Eynatten, Maximilian; Mattheus, Michaela; Johansen, Odd Erik; Woerle, Hans J.; Broedl, Uli C.; Zinman, Bernard (2016-07-28). "Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes". New England Journal of Medicine. 375 (4): 323–334. doi:10.1056/nejmoa1515920. ISSN 0028-4793. PMID 27299675.
- Li, Ting; Wu, Hong Mei; Wang, Feng; Huang, Chang Quan; Yang, Ming; Dong, Bi Rong; Liu, Guan J (2011-06-15). Cochrane Kidney and Transplant Group (ed.). "Education programmes for people with diabetic kidney disease". Cochrane Database of Systematic Reviews (6): CD007374. doi:10.1002/14651858.CD007374.pub2. PMID 21678365.
- Shlipak M (2011-03-15). "Clinical Evidence Handbook: Diabetic Nephropathy: Preventing Progression – American Family Physician". American Family Physician. 83 (6): 732. Retrieved 2015-06-27.
- Zimmet, Paul; Alberti, K. G. M. M.; Shaw, Jonathan (December 2001). "Global and societal implications of the diabetes epidemic". Nature. 414 (6865): 782–787. Bibcode:2001Natur.414..782Z. doi:10.1038/414782a. ISSN 0028-0836. PMID 11742409. S2CID 4384190.
- Cameron, Adrian J; Zimmet, Paul Z; Atkins, Robert C; Shaw, Jonathan E (2007). "The Australian Diabetes, Obesity and Lifestyle Study – Profiling Diabetes and Cardiovascular Disease Risk in the Nation". European Endocrinology (2): 20. doi:10.17925/ee.2007.00.02.20. ISSN 1758-3772.
- Trivedi, Hariprasad S.; Pang, Michael M.H.; Campbell, Anne; Saab, Paulette (April 2002). "Slowing the progression of chronic renal failure: Economic benefits and patients' perspectives". American Journal of Kidney Diseases. 39 (4): 721–13. doi:10.1053/ajkd.2002.31990. ISSN 0272-6386. PMID 11920337.
- Disease, Ethnicity & (2018-10-17). "Correction: Ethn Dis. 2010;20:[Suppl 1]:S1-60-S1-64". Ethnicity & Disease. 28 (4): 586. doi:10.18865/ed.28.4.586. ISSN 1945-0826. PMC 6200305. PMID 30405305.
Further reading
- "Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: an updated meta-analysis". www.crd.york.ac.uk. Retrieved 2015-07-02.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (January 2005). "Diabetic nephropathy: diagnosis, prevention, and treatment". Diabetes Care. 28 (1): 164–76. doi:10.2337/diacare.28.1.164. PMID 15616252.
- Tziomalos K, Athyros VG (2015). "Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis". The Review of Diabetic Studies. 12 (1–2): 110–8. doi:10.1900/RDS.2015.12.110. PMC 5397986. PMID 26676664.
- Kume S, Koya D, Uzu T, Maegawa H (2014). "Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy". BioMed Research International. 2014: 315494. doi:10.1155/2014/315494. PMC 4122096. PMID 25126552.
- Doshi SM, Friedman AN (August 2017). "Diagnosis and Management of Type 2 Diabetic Kidney Disease". Clinical Journal of the American Society of Nephrology. 12 (8): 1366–1373. doi:10.2215/CJN.11111016. PMC 5544517. PMID 28280116.
External links
| Classification | D |
|---|---|
| External resources |
| Disease of the kidney glomerules | |||||||
|---|---|---|---|---|---|---|---|
| Primarily nephrotic |
| ||||||
| Primarily nephritic, RPG |
| ||||||
| General | |||||||
| Diabetes | |
|---|---|
| Types |
|
| Blood tests |
|
| Management |
|
| Complications |
|
| Advocacy & Organizations | |
| Other | |