| Revision as of 21:44, 12 December 2010 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Ch← Previous edit | Latest revision as of 16:26, 20 January 2025 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers75,010 edits rm baby talk | ||
| (236 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Iodo-derivative of fluorone used as a pink dye}} | |||
| {{Use dmy dates|date=January 2025}} | |||
| {{redirect|Red 3|the techno release called Red Three|Dave Clarke (DJ)}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | | verifiedrevid = 442926733 | ||
| ⚫ | | |
||
| ⚫ | | Name = Erythrosine | ||
| ⚫ | | |
||
| | |
| ImageFile = Erythrosine.svg | ||
| ⚫ | | ImageSize = 230px | ||
| ⚫ | | |
||
| ⚫ | | ImageName = Erythrosine | ||
| ⚫ | | |
||
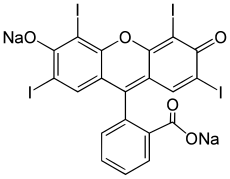
| ⚫ | | IUPACName = 2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid | ||
| ⚫ | | |
||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 3144 | | ChemSpiderID = 3144 | ||
| | |
| ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | |
| ChEMBL = 1332616 | ||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | UNII = 8TL7LH93FM | |||
| | InChI = 1/C20H8I4O5/c21-11-5-9-17(13(23)15(11)25)28-18-10(6-12(22)16(26)14(18)24)20(9)8-4-2-1-3-7(8)19(27)29-20/h1-6,25-26H | | InChI = 1/C20H8I4O5/c21-11-5-9-17(13(23)15(11)25)28-18-10(6-12(22)16(26)14(18)24)20(9)8-4-2-1-3-7(8)19(27)29-20/h1-6,25-26H | ||
| | InChIKey = OALHHIHQOFIMEF-UHFFFAOYAB | | InChIKey = OALHHIHQOFIMEF-UHFFFAOYAB | ||
| Line 18: | Line 25: | ||
| | StdInChIKey = OALHHIHQOFIMEF-UHFFFAOYSA-N | | StdInChIKey = OALHHIHQOFIMEF-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 16423-68-0 | | CASNo = 16423-68-0 | ||
| | |
| SMILES = C(=O)C1=CC=CC=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C1C=C(I)C()=C2I.. | ||
| | |
| PubChem = 3259 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>20</sub>H<sub>6</sub>I<sub>4</sub>Na<sub>2</sub>O<sub>5</sub> | ||
| | |
| MolarMass = 879.86 g/mol | ||
| | |
| Density = | ||
| | MeltingPtC = 142 to 144 | |||
| | MeltingPt = | |||
| | MeltingPt_ref = <ref>{{cite web | title = Erythrosine B product description | url = http://www.chemicalbook.com/ChemicalProductProperty_US_CB0393696.aspx | work = The Chemical Book }}</ref> | |||
| | |
| BoilingPt = | ||
| }} | }} | ||
| |Section3={{Chembox Hazards | |||
| | NFPA-H = 2 | |||
| | NFPA-F = 1 | |||
| | NFPA-R = 0 | |||
| }} | }} | ||
| }} | |||
| '''Erythrosine''', also known as '''E127''' and '''Red No. 3''', is an ], specifically a derivative of ]. It is a red-pink ] used for ], ], ], pet products, and diverse industrial colorings.<ref name="pubchem">{{cite web |title=Erythrosine sodium anhydrous |url=https://pubchem.ncbi.nlm.nih.gov/compound/16423-68-0 |publisher=PubChem, US National Library of Medicine |access-date=17 January 2025 |date=11 January 2025}}</ref><ref name=Ullmann>{{Ullmann |last1=Lyday |first1=Phyllis A |last2=Kaiho |first2=Tatsuo |title=Iodine and Iodine Compounds |doi=10.1002/14356007.a14_381.pub2 }}</ref> It is the disodium ] of 2,4,5,7-tetraiodo].<ref name=pubchem/> | |||
| ==History== | |||
| {{Section-stub|the article would benefit from details on its phaseout as a textile dye|date=January 2025|small=no}} | |||
| The colorant was discovered by Swiss chemist Karl Kussmaul at the ] in 1876 and soon commercialized by local ] company for dyeing wool and silk.<ref>{{Cite book |url=https://books.google.com/books?id=djEkDZp7lEgC&pg=PA1171 |title=Le Moniteur scientifique du Docteur Quesneville: journal des sciences pures et appliquées |date=1878 |publisher=Le Moniteur scientifique |pages=1171 |language=fr}}</ref><ref>{{Cite book |url=https://books.google.com/books?id=lY45AQAAIAAJ&pg=PA48 |title=A Dictionary of the Coal Tar Colours |date=1892 |publisher=Heywood and Company |pages=48 |language=en}}</ref> | |||
| Its use as a food dye was legalized in the US by the ] of 1906.<ref>{{Cite book |last=Deshpande |first=S. S. |url=https://books.google.com/books?id=xc39DwAAQBAJ&pg=PA227 |title=Handbook of Food Toxicology |date=2002-08-29 |publisher=CRC Press |isbn=978-0-203-90896-9 |pages=227 |language=en}}</ref> By early 1920s, it was produced mainly for the food industry,<ref>{{Cite book |last= |first= |url=https://books.google.com/books?id=bfhIAQAAIAAJ&pg=RA2-PA56 |title=The Emergency Tariff and Its Effect on Cattle and Beef, Sheep and Mutton, Wool, Pork, and Miscellaneous Meats |date=1922 |publisher=U.S. Government Printing Office |language=en}}</ref> with {{Convert|2,170|lb|t}} made in America in 1924,<ref>{{cite book |author1=United States Tariff Commission |author1-link=United States International Trade Commission |title=Census of Dyes and Other Synthetic Organic Chemicals |date=1925 |publisher=] |url=https://books.google.com/books?id=z0SpfTX1taEC&pg=RA1-PA64 |publication-place=Washington, D.C. |page=64 |access-date=17 January 2025 |series=Tariff Information Series |lang=en}}</ref> rising to {{Convert|9,468|lb|t|2}} in 1938<ref>{{cite periodical |last1=Taylor |first1=J.N. |title=Organic Chemicals |periodical=World Trade Notes on Chemicals and Allied Products |date=13 July 1940 |volume=14 |issue=28 |page=456 |url=https://books.google.com/books?id=jdseHwBxG5YC&pg=PA456 |access-date=17 January 2025 |publisher=] |location=Washington, D.C. |language=en}}</ref> and approximately 50 tons in 1967.<ref>{{Cite book |last=Gunther |first=Francis A. |url=https://books.google.com/books?id=ewggAAAAMAAJ&q=erythrosine+tons |title=Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment |date=1983-05-11 |publisher=Springer New York |isbn=978-0-387-90851-9 |language=en}}</ref> | |||
| ==Production== | |||
| '''Erythrosine''', also known as Red No. 3, is an ], specifically a derivative of ]. It is cherry-pink synthetic, primarily used for ].<ref name=Ullmann>Phyllis A. Lyday "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim</ref> It is the disodium ] of 2,4,5,7-tetraiodo]. Its maximum absorbance is at 530 ]<ref></ref> in an aqueous solution, and it is subject to ].{{Citation needed|date=February 2007}} | |||
| Erythrosine is synthesized from ] and ], which are processed into ]. Fluorescein then undergoes iodination, which give the bright red dye. | |||
| ==Uses== | ==Uses== | ||
| It is used as a ],<ref>{{cite journal | vauthors = Chequer FM, Venâncio VP, Bianchi ML, Antunes LM | title = Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells | journal = Food and Chemical Toxicology | volume = 50 | issue = 10 | pages = 3447–51 | date = October 2012 | pmid = 22847138 | doi = 10.1016/j.fct.2012.07.042 | doi-access = free }}</ref> ] ink,<ref>{{cite book |title=Synthetic Dyes in Biology, Medicine And Chemistry |last=Gurr |first=Edward | name-list-style = vanc |publisher=elsevier|year=2012|pages=197, 198}}</ref> biological stain,<ref name=":0" /> ] disclosing agent,<ref>{{cite journal | vauthors = Wood S, Metcalf D, Devine D, Robinson C | title = Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms | journal = The Journal of Antimicrobial Chemotherapy | volume = 57 | issue = 4 | pages = 680–4 | date = April 2006 | pmid = 16464894 | doi = 10.1093/jac/dkl021 | doi-access = free }}</ref> ] medium,<ref name=":0">{{cite book |title=Dictionary of Analytical Reagents |publisher=CRC Press |year=1993 |isbn= 978-0-412-35150-1 |pages=474 }}</ref> sensitizer for ] photographic films, and visible light photoredox catalyst.<ref>{{cite journal | vauthors = Rogers DA, Brown RG, Brandeburg ZC, Ko EY, Hopkins MD, LeBlanc G, Lamar AA | title = Organic Dye-Catalyzed, Visible-Light Photoredox Bromination of Arenes and Heteroarenes Using N-Bromosuccinimide | journal = ACS Omega | volume = 3 | issue = 10 | pages = 12868–12877 | date = October 2018 | pmid = 31458011 | pmc = 6644467 | doi = 10.1021/acsomega.8b02320 }}</ref> | |||
| It is used as a ], in ] inks, as a biological stain, a ] disclosing agent and a ] medium. Erythrosine is commonly used in sweets such as some candies and popsicles, and even more widely used in cake-decorating gels. It is also used to color ] shells.<ref></ref><ref>Blue Diamond Ultra Premium Blend Mixed Nuts, distributed by Diamond Foods, Inc. Stockton, CA</ref> As a food additive, it has the ] E127. | |||
| Erythrosine is commonly used in sweets, such as some candies, ] and ], and in cake-decorating gels.<ref name="fda-red3">{{cite web |title=FD&C Red No. 3 |url=https://www.fda.gov/industry/color-additives/fdc-red-no-3 |publisher=US Food and Drug Administration |access-date=15 January 2025 |language=en |date=15 January 2025}}</ref> It was also used to color ] shells.<ref>{{Cite web |last=Day |first=Chris |date=2023-01-16 |title=The Almost-Forgotten Era Of Red Pistachios |url=https://www.thedailymeal.com/1168708/the-almost-forgotten-era-of-red-pistachios/ |access-date=2023-11-17 |website=The Daily Meal |language=en-US}}</ref> As a food additive, it has the ] E127.<ref name="efsa">{{cite journal |author=EFSA Panel on Additives and Products or Substances used in Animal Feed |author1-link=European Food Safety Authority |title=Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive |journal=EFSA Journal |date=January 2011 |volume=9 |issue=1 |pages=1854 |doi=10.2903/j.efsa.2011.1854 |url=https://phaidra.univie.ac.at/o:245195 |quote=Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries}}</ref> | |||
| While commonly in most countries of the world, erythrosine is less commonly used in the United States with ] (Red #40) being generally used instead. However, Allura Red AC is banned in many European countries because it is an ], despite the fact that it has fewer known health risks than erythrosine.{{Citation needed|date=April 2010}} | |||
| ==Safety assessment and regulation== | |||
| As a result of efforts begun in the 1970s, in 1990 the U.S. FDA had instituted a partial ban on erythrosine, citing research that high doses have been found to cause cancer in rats.<ref>, ''The Washington Post'', February 7, 1990</ref> In June of 2008, the ] (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States.<ref>, CBS News, June 3, 2008</ref> | |||
| Concerns were raised by laboratory studies in the late ] that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by ]" occurring with 4% erythrosine.<ref>{{cite journal |vauthors = Jennings AS, Schwartz SL, Balter NJ, Gardner D, Witorsch RJ |title = Effects of oral erythrosine (2′,4′,5′,7′-tetraiodofluorescein) on the pituitary-thyroid axis in rats |journal = Toxicology and Applied Pharmacology |volume = 103 |issue = 3 |pages = 549–56 |date = May 1990 |pmid = 2160137 |doi = 10.1016/0041-008x(90)90327-q |doi-access = free}}</ref><ref name="borz">{{cite journal |vauthors=Borzelleca JF, Capen CC, Hallagan JB |title=Lifetime toxicity/carcinogenicity study of FD & C Red No. 3 (erythrosine) in rats |journal=Food and Chemical Toxicology|volume=25 |issue=10 |pages=723–33 |date=October 1987 |pmid=2824305 |doi=10.1016/0278-6915(87)90226-2 |url=https://www.sciencedirect.com/science/article/abs/pii/0278691587902262}}</ref> Toxicology tests combined with a review of other reported studies concluded that erythrosine is non-] and any tumor growth results from a non-genotoxic mechanism.<ref name=fda-red3/><ref>{{cite journal |vauthors = Lin GH, Brusick DJ |title = Mutagenicity studies on FD&C red No.3 |journal = Mutagenesis |volume = 1 |issue = 4 |pages = 253–9 |date = July 1986 |pmid = 2457780 |doi = 10.1093/mutage/1.4.253 }}</ref> | |||
| In the United States, laboratory evidence of ] by extremely high doses of erythrosine renders it as "unsafe" under federal law by a provision called the ], despite conclusions by the federal ] and Cancer Assessment Committee that the risk of developing cancer in humans is unlikely at the low erythrosine levels consumed as a food color.<ref name=fda-red3/><ref name="fr-fda">{{cite web |title=Color Additive Petition From Center for Science in the Public Interest, et al.; Request To Revoke Color Additive Listing for Use of FD&C Red No. 3 in Food and Ingested Drugs |url=https://www.federalregister.gov/documents/2025/01/16/2025-00830/color-additive-petition-from-center-for-science-in-the-public-interest-et-al-request-to-revoke-color |publisher=Federal Register, US Food and Drug Administration |access-date=17 January 2025 |date=15 January 2025}}</ref> | |||
| ⚫ | ==Synonyms== |
||
| ⚫ | Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD |
||
| Throughout the early ], the ] and several national food safety agencies permitted use of erythrosine as a color additive under restrictions that it be used in amounts below ] levels for certain foods, such as for packaged cherries; countries having restricted-use provisions were the European Union, United States, Canada, and Australia/New Zealand.<ref name=efsa/><ref name=fr-fda/><ref name="who">{{cite web |title=FD and C RED No. 3 |url=https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/503 |publisher=Joint FAO/WHO Expert Committee on Food Additives, World Health Organization |access-date=17 January 2025 |date=2018}}</ref><ref name="cbc">{{cite news |title=Red dye No. 3 banned from foods, U.S. regulator says |url=https://www.cbc.ca/news/health/red-dye-3-1.7431754 |access-date=17 January 2025 |work=CBC News |agency=The Associated Press |date=15 January 2025}}</ref><ref>{{Cite web |publisher= Office Parliamentary Counsel; Locked Bag 30 Kingston ACT 2604, ] |title=Australia New Zealand Food Standards Code – Schedule 8 – Food additive names and code numbers (for statement of ingredients) |date=2021-03-26 |url=https://www.legislation.gov.au/F2015L00478/latest/text |access-date=2024-09-19}}</ref> | |||
| ⚫ | 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein |
||
| Since 1994,<ref>{{Citation |title=European Parliament and Council Directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs |date=1994-06-30 |volume=237 |url=https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:31994L0036 |access-date=2025-01-16 |language=en}}</ref> the ] only allows erythrosine in processed cherries<ref name=efsa/> and pet foods.<ref>{{cite journal |author=EFSA Panel on Additives and Products or Substances used in Animal Feed |title=Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish |journal=EFSA Journal |date=2015 |volume=13 |issue=9 |page=4233 |doi=10.2903/j.efsa.2015.4233|author1-link=European Food Safety Authority |doi-access=free |hdl=2434/559021 |hdl-access=free }}</ref><ref>{{cite journal |author=EFSA Panel on Additives and Products or Substances used in Animal Feed|title=Safety of erythrosine for ornamental fish |journal=EFSA Journal |date=2019 |volume=17 |issue=5 |page=5699 |doi=10.2903/j.efsa.2019.5699|pmid=32626322 |pmc=7009114 |author1-link=European Food Safety Authority }}</ref> It is also allowed in ] up to 25 ].<ref>{{Cite report |author=SCCS (Scientific Committee on Consumer Safety) |author-link=Scientific Committee on Consumer Safety |date=22 January 2010 |title=Opinion on CI 45 430 (erythrosine) |lang=en |url=https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_030.pdf |publisher=] |doi=10.2772/24102 |isbn=978-92-79-12741-0 |issn=1831-4767 |id=ND-AQ-09-013-EN-N |doi-access=free }}</ref> In the ], erythrosine is also allowed for coloring eggshells. It is not permitted to be sold directly to consumers.<ref>{{cite web |url=https://www.npr.org/2021/10/15/1046348573/sprinklegate-sinks-a-u-k-bakerys-top-sellers-after-topping-is-found-to-be-illega |title='Sprinklegate' sinks a U.K. Bakery's top sellers after topping is found to be illegal |work=NPR |date=15 October 2021 |last1=Chappell |first1=Bill |access-date=20 January 2025 }}</ref> | |||
| In the United States, the use of erythrosine in cosmetics, ] drugs, some foods, and in all uses as its ] variant have been banned by the ] (FDA) since 1990.<ref name="lat_1990_fda_ban">{{cite news |title=FDA Bans Some Uses of Red No. 3 Additive |url=https://www.latimes.com/archives/la-xpm-1990-01-29-fi-917-story.html |access-date=15 January 2025 |work=] |agency=] |date=29 January 1990}}</ref> In January 2025, the FDA banned the use of erythrosine in all foods and ingested drugs, with enforcement beginning on 15 January 2027 and 18 January 2028, respectively.<ref name=fda-red3/> An October 2023 bill passed in the state of ] also banned the use of erythrosine in foods (along with ], ], and ]), with enforcement beginning on 1 January 2027.<ref>{{Cite act |title=The California Food Safety Act |year=2023 |type=AB |index=418 |legislature=] |last1=Gabriel |first1=Jesse |author-link1=Jesse Gabriel |last2=Wicks |first2=Buffy |author-link2=Buffy Wicks |last3=McCarty |first3=Kevin |author-link3=Kevin McCarty |last4=Weber |first4=Akilah |author-link4=Akilah Weber |display-authors=1 |orig-date=Approved by Governor 7 October 2023 |url=https://leginfo.legislature.ca.gov/faces/billStatusClient.xhtml?bill_id=202320240AB418 }}</ref> | |||
| In 2025, ] stated that erythrosine "does not pose a health risk to the general Canadian population at the levels set out in the ''List of Permitted Food Colours''."<ref name=cbc/> | |||
| ⚫ | ==Synonyms== | ||
| ⚫ | Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD&C Red No. 3; E127; | ||
| ⚫ | 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein sodium salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I. Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiroxanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.<ref name=pubchem/> | ||
| ==Classification== | ==Classification== | ||
| Line 48: | Line 80: | ||
| * ] Red No. 3 | * ] Red No. 3 | ||
| * ] E127 (Food Red 14) | * ] E127 (Food Red 14) | ||
| * ] no. 45430 (Acid Red 51) | * ] no. 45430 (Acid Red 51) | ||
| * ] No. 1697 | * ] No. 1697 | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Authority control}} | |||
| ==External links== | |||
| * | |||
| * | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 16:26, 20 January 2025
Iodo-derivative of fluorone used as a pink dye"Red 3" redirects here. For the techno release called Red Three, see Dave Clarke (DJ).
 | |
| Names | |
|---|---|
| IUPAC name 2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.036.390 |
| E number | E127 (colours) |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H6I4Na2O5 |
| Molar mass | 879.86 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Erythrosine, also known as E127 and Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is a red-pink dye used for food coloring, cosmetics, hair coloring, pet products, and diverse industrial colorings. It is the disodium salt of 2,4,5,7-tetraiodofluorescein.
History
| This section needs expansion with: the article would benefit from details on its phaseout as a textile dye. You can help by adding to it. (January 2025) |
The colorant was discovered by Swiss chemist Karl Kussmaul at the University of Basel in 1876 and soon commercialized by local Bindschedler & Busch company for dyeing wool and silk.
Its use as a food dye was legalized in the US by the Pure Food and Drug Act of 1906. By early 1920s, it was produced mainly for the food industry, with 2,170 pounds (0.98 t) made in America in 1924, rising to 9,468 pounds (4.29 t) in 1938 and approximately 50 tons in 1967.
Production
Erythrosine is synthesized from phenol and phthalic anhydride, which are processed into fluorescein. Fluorescein then undergoes iodination, which give the bright red dye.
Uses
It is used as a food coloring, printing ink, biological stain, dental plaque disclosing agent, radiopaque medium, sensitizer for orthochromatic photographic films, and visible light photoredox catalyst.
Erythrosine is commonly used in sweets, such as some candies, ice pops and cherries, and in cake-decorating gels. It was also used to color pistachio shells. As a food additive, it has the E number E127.
Safety assessment and regulation
Concerns were raised by laboratory studies in the late 20th century that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by TSH" occurring with 4% erythrosine. Toxicology tests combined with a review of other reported studies concluded that erythrosine is non-genotoxic and any tumor growth results from a non-genotoxic mechanism.
In the United States, laboratory evidence of carcinogenicity by extremely high doses of erythrosine renders it as "unsafe" under federal law by a provision called the Delaney Clause, despite conclusions by the federal Center for Food Safety and Applied Nutrition and Cancer Assessment Committee that the risk of developing cancer in humans is unlikely at the low erythrosine levels consumed as a food color.
Throughout the early 21st century, the World Health Organization and several national food safety agencies permitted use of erythrosine as a color additive under restrictions that it be used in amounts below acceptable daily intake levels for certain foods, such as for packaged cherries; countries having restricted-use provisions were the European Union, United States, Canada, and Australia/New Zealand.
Since 1994, the European Food Safety Authority only allows erythrosine in processed cherries and pet foods. It is also allowed in toothpaste up to 25 ppm. In the United Kingdom, erythrosine is also allowed for coloring eggshells. It is not permitted to be sold directly to consumers.
In the United States, the use of erythrosine in cosmetics, topical drugs, some foods, and in all uses as its lake variant have been banned by the Food and Drug Administration (FDA) since 1990. In January 2025, the FDA banned the use of erythrosine in all foods and ingested drugs, with enforcement beginning on 15 January 2027 and 18 January 2028, respectively. An October 2023 bill passed in the state of California also banned the use of erythrosine in foods (along with brominated vegetable oil, potassium bromate, and propylparaben), with enforcement beginning on 1 January 2027.
In 2025, Health Canada stated that erythrosine "does not pose a health risk to the general Canadian population at the levels set out in the List of Permitted Food Colours."
Synonyms
Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD&C Red No. 3; E127; 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein sodium salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I. Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiroxanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.
Classification
It is listed under the following number systems:
- FD&C Red No. 3
- E number E127 (Food Red 14)
- Colour Index no. 45430 (Acid Red 51)
- Bureau of Indian Standards No. 1697
References
- "Erythrosine B product description". The Chemical Book.
- ^ "Erythrosine sodium anhydrous". PubChem, US National Library of Medicine. 11 January 2025. Retrieved 17 January 2025.
- Lyday, Phyllis A; Kaiho, Tatsuo. "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_381.pub2. ISBN 978-3-527-30673-2.
- Le Moniteur scientifique du Docteur Quesneville: journal des sciences pures et appliquées (in French). Le Moniteur scientifique. 1878. p. 1171.
- A Dictionary of the Coal Tar Colours. Heywood and Company. 1892. p. 48.
- Deshpande, S. S. (29 August 2002). Handbook of Food Toxicology. CRC Press. p. 227. ISBN 978-0-203-90896-9.
- The Emergency Tariff and Its Effect on Cattle and Beef, Sheep and Mutton, Wool, Pork, and Miscellaneous Meats. U.S. Government Printing Office. 1922.
- United States Tariff Commission (1925). Census of Dyes and Other Synthetic Organic Chemicals. Tariff Information Series. Washington, D.C.: Government Printing Office. p. 64. Retrieved 17 January 2025.
- Taylor, J.N. (13 July 1940). "Organic Chemicals". World Trade Notes on Chemicals and Allied Products. Vol. 14, no. 28. Washington, D.C.: Department of Commerce Chemical Division. p. 456. Retrieved 17 January 2025.
- Gunther, Francis A. (11 May 1983). Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment. Springer New York. ISBN 978-0-387-90851-9.
- Chequer FM, Venâncio VP, Bianchi ML, Antunes LM (October 2012). "Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells". Food and Chemical Toxicology. 50 (10): 3447–51. doi:10.1016/j.fct.2012.07.042. PMID 22847138.
- Gurr E (2012). Synthetic Dyes in Biology, Medicine And Chemistry. elsevier. pp. 197, 198.
- ^ Dictionary of Analytical Reagents. CRC Press. 1993. p. 474. ISBN 978-0-412-35150-1.
- Wood S, Metcalf D, Devine D, Robinson C (April 2006). "Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms". The Journal of Antimicrobial Chemotherapy. 57 (4): 680–4. doi:10.1093/jac/dkl021. PMID 16464894.
- Rogers DA, Brown RG, Brandeburg ZC, Ko EY, Hopkins MD, LeBlanc G, Lamar AA (October 2018). "Organic Dye-Catalyzed, Visible-Light Photoredox Bromination of Arenes and Heteroarenes Using N-Bromosuccinimide". ACS Omega. 3 (10): 12868–12877. doi:10.1021/acsomega.8b02320. PMC 6644467. PMID 31458011.
- ^ "FD&C Red No. 3". US Food and Drug Administration. 15 January 2025. Retrieved 15 January 2025.
- Day, Chris (16 January 2023). "The Almost-Forgotten Era Of Red Pistachios". The Daily Meal. Retrieved 17 November 2023.
- ^ EFSA Panel on Additives and Products or Substances used in Animal Feed (January 2011). "Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive". EFSA Journal. 9 (1): 1854. doi:10.2903/j.efsa.2011.1854.
Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries
- Jennings AS, Schwartz SL, Balter NJ, Gardner D, Witorsch RJ (May 1990). "Effects of oral erythrosine (2′,4′,5′,7′-tetraiodofluorescein) on the pituitary-thyroid axis in rats". Toxicology and Applied Pharmacology. 103 (3): 549–56. doi:10.1016/0041-008x(90)90327-q. PMID 2160137.
- Borzelleca JF, Capen CC, Hallagan JB (October 1987). "Lifetime toxicity/carcinogenicity study of FD & C Red No. 3 (erythrosine) in rats". Food and Chemical Toxicology. 25 (10): 723–33. doi:10.1016/0278-6915(87)90226-2. PMID 2824305.
- Lin GH, Brusick DJ (July 1986). "Mutagenicity studies on FD&C red No.3". Mutagenesis. 1 (4): 253–9. doi:10.1093/mutage/1.4.253. PMID 2457780.
- ^ "Color Additive Petition From Center for Science in the Public Interest, et al.; Request To Revoke Color Additive Listing for Use of FD&C Red No. 3 in Food and Ingested Drugs". Federal Register, US Food and Drug Administration. 15 January 2025. Retrieved 17 January 2025.
- "FD and C RED No. 3". Joint FAO/WHO Expert Committee on Food Additives, World Health Organization. 2018. Retrieved 17 January 2025.
- ^ "Red dye No. 3 banned from foods, U.S. regulator says". CBC News. The Associated Press. 15 January 2025. Retrieved 17 January 2025.
- "Australia New Zealand Food Standards Code – Schedule 8 – Food additive names and code numbers (for statement of ingredients)". Office Parliamentary Counsel; Locked Bag 30 Kingston ACT 2604, Food Standards Australia New Zealand. 26 March 2021. Retrieved 19 September 2024.
- European Parliament and Council Directive 94/36/EC of 30 June 1994 on colours for use in foodstuffs, vol. 237, 30 June 1994, retrieved 16 January 2025
- EFSA Panel on Additives and Products or Substances used in Animal Feed (2015). "Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish". EFSA Journal. 13 (9): 4233. doi:10.2903/j.efsa.2015.4233. hdl:2434/559021.
- EFSA Panel on Additives and Products or Substances used in Animal Feed (2019). "Safety of erythrosine for ornamental fish". EFSA Journal. 17 (5): 5699. doi:10.2903/j.efsa.2019.5699. PMC 7009114. PMID 32626322.
- SCCS (Scientific Committee on Consumer Safety) (22 January 2010). Opinion on CI 45 430 (erythrosine) (PDF) (Report). European Commission. doi:10.2772/24102. ISBN 978-92-79-12741-0. ISSN 1831-4767. ND-AQ-09-013-EN-N.
- Chappell, Bill (15 October 2021). "'Sprinklegate' sinks a U.K. Bakery's top sellers after topping is found to be illegal". NPR. Retrieved 20 January 2025.
- "FDA Bans Some Uses of Red No. 3 Additive". Los Angeles Times. Associated Press. 29 January 1990. Retrieved 15 January 2025.
- Gabriel, Jesse; et al. (2023) . The California Food Safety Act (AB 418). California State Legislature.