| Revision as of 20:23, 3 February 2011 editWolfmanSF (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers156,372 edits →Analogs and derivatives: mention 2-N analogs← Previous edit | Latest revision as of 17:38, 21 February 2024 edit undoRoger Burger (talk | contribs)49 editsm More correct structure of heme B. | ||
| (260 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Organic ring compound (C₄H₄NH)}} | |||
| {{Chembox | {{Chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 408974067 | |||
| | verifiedrevid = 464377778 | |||
| | Name = Pyrrole | | Name = Pyrrole | ||
| | ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | ImageFileL1 = Pyrrole-2D-numbered.svg | |||
| | ImageFileL1 = Pyrrole-2D-full.svg | |||
| | ImageSizeL1 = 100 | |||
| | |
| ImageNameL1 = Explicit structural formula of pyrrole, with aromaticity indicated by dashed bonds | ||
| | ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| | ImageFileR1 = Pyrrole-2D-full.svg | |||
| | ImageFileR1 = Pyrrole-2D-numbered.svg | |||
| | ImageSizeR1 = 100 | |||
| | |
| ImageNameR1 = Numbered skeletal formula of pyrrole | ||
| | ImageFileL2 = Pyrrole-CRC-MW-3D-balls-A.png | | ImageFileL2 = Pyrrole-CRC-MW-3D-balls-A.png | ||
| | |
| ImageNameL2 = Ball-and-stick model of the pyrrole molecule | ||
| | ImageFileR2 = Pyrrole-CRC-MW-3D-vdW.png | | ImageFileR2 = Pyrrole-CRC-MW-3D-vdW.png | ||
| | |
| ImageNameR2 = Space-filling model of the pyrrole molecule | ||
| | PIN = 1''H''-Pyrrole<ref>{{cite book |author=] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=] |pages=141 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> | |||
| | IUPACName = 1''H''-Pyrrole | |||
| | OtherNames = {{Unbulleted list|Azole|Imidole<ref name="crc97">{{cite book | author=William M. Haynes | title=CRC Handbook of Chemistry and Physics | edition=97th | year=2016 | publisher=CRC Press | location=Boca Raton | isbn=978-1-4987-5429-3 | pages=3–478}}</ref>}} | |||
| | Section1 = {{Chembox Identifiers | |||
| |Section1={{Chembox Identifiers | |||
| | CASNo = 109-97-7 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo = 109-97-7 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | |
| PubChem = 8027 | ||
| | ChemSpiderID = 7736 | |||
| | PubChem_Ref = {{Pubchemcite|correct|PubChem}} | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 7736 | |||
| | UNII = 86S1ZD6L2C | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 86S1ZD6L2C | |||
| | UNNumber = 1992, 1993 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | |
| EINECS = 203-724-7 | ||
| | |
| RTECS = UX9275000 | ||
| | |
| SMILES = N1C=CC=C1 | ||
| | |
| SMILES1 = 1cccc1 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | SMILES1 = c1ccc1 | |||
| | |
| ChEBI = 19203 | ||
| | |
| ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 16225 | | ChEMBL = 16225 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C4H5N/c1-2-4-5-3-1/h1-5H | | StdInChI = 1S/C4H5N/c1-2-4-5-3-1/h1-5H | ||
| | |
| InChI = 1/C4H5N/c1-2-4-5-3-1/h1-5H | ||
| | |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = KAESVJOAVNADME-UHFFFAOYSA-N | | StdInChIKey = KAESVJOAVNADME-UHFFFAOYSA-N | ||
| | |
| Beilstein = 1159 | ||
| | |
| Gmelin = 1705}} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| C = 4 | ||
| | |
| H = 5 | ||
| | |
| N = 1 | ||
| | |
| Density = 0.967 g cm<sup>−3</sup> | ||
| | MeltingPtC = −23 | |||
| | Density = 0.967 g cm<sup>-3</sup> | |||
| | BoilingPtC = 129 to 131 | |||
| | MeltingPtC = −23 | |||
| | VaporPressure = 7 mmHg at 23 °C | |||
| | BoilingPtCL = 129 | |||
| | pKa = 17.5 (for the N−H proton) | |||
| | BoilingPtCH = 131 | |||
| | pKb = 13.6 (p''K''<sub>a</sub> 0.4 for ]) | |||
| | VaporPressure = 7 mmHg at 23 °C | |||
| | MagSus = {{val|-47.6e-6|u=cm<sup>3</sup> mol<sup>−1</sup>}} | |||
| | Viscosity = 0.001225 Pa s}} | |||
| | Viscosity = 0.001225 Pa s}} | |||
| | Section3 = {{Chembox Thermochemistry | |||
| |Section3={{Chembox Thermochemistry | |||
| | DeltaHf = 108.2 kJ mol<sup>-1</sup> (gas) | |||
| | |
| DeltaHf = 108.2 kJ mol<sup>−1</sup> (gas) | ||
| | |
| DeltaHc = 2242 kJ mol<sup>−1</sup> | ||
| | HeatCapacity = 1.903 J K<sup>−1</sup> mol<sup>−1</sup> | |||
| }} | |||
| }} | |||
| | Section4 = {{Chembox Hazards | |||
| |Section4={{Chembox Hazards | |||
| | NFPA-H = 2 | |||
| | ExternalSDS = | |||
| | NFPA-F = 2 | |||
| | |
| NFPA-H = 2 | ||
| | |
| NFPA-F = 2 | ||
| | NFPA-R = 0 | |||
| | Autoignition = 550°C | |||
| | |
| FlashPtC = 33.33 | ||
| | AutoignitionPtC = 550 | |||
| }} | |||
| | ExploLimits = 3.1–14.8% | |||
| }} | }} | ||
| |Section8={{Chembox Related | |||
| '''Pyrrole''' is a ] ] ], a five-membered ring with the ] ]<sub>4</sub>]<sub>4</sub>]H.<ref>{{cite book | |||
| | OtherCompounds = ], ], ], ]}} | |||
| | author = Loudon, Marc G. | |||
| }} | |||
| '''Pyrrole''' is a ], ], ], a five-membered ] with the ] {{chem2|C4H4NH}}.<ref>{{cite book | |||
| | last = Loudon | |||
| | first = Marc G. | |||
| | year = 2002 | | year = 2002 | ||
| | title = Organic Chemistry | | title = Organic Chemistry | ||
| | chapter = Chemistry of Naphthalene and the Aromatic Heterocycles |
| chapter = Chemistry of Naphthalene and the Aromatic Heterocycles | ||
| | edition = |
| edition = 4th | ||
| | pages = 1135–1136 | | pages = 1135–1136 | ||
| | publisher = Oxford University Press | | publisher = Oxford University Press | ||
| | location = New York | | location = New York | ||
| | isbn = 0-19-511999- |
| isbn = 978-0-19-511999-2 | ||
| }}</ref> |
}}</ref> It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, {{chem2|C4H4NCH3}}. ], a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as ].<ref>{{cite book |last1=Cox |first1=Michael |last2=Lehninger |first2=Albert L. |last3=Nelson |first3=David R. |title=Lehninger Principles of Biochemistry |publisher=Worth Publishers |location=New York |year=2000 |isbn=978-1-57259-153-0 |url-access=registration |url=https://archive.org/details/lehningerprincip01lehn }}</ref> | ||
| Pyrroles are components of more complex macrocycles, including the ]s of ], the ]s, bacteriochlorins, ] |
Pyrroles are components of more complex macrocycles, including the ]s and products derived therefrom, including ]s of ], the ]s, bacteriochlorins, and ]s.<ref name="Jonas Jusélius and Dage Sundholm 2000 2145–2151">{{cite journal | title = The aromatic pathways of porphins, chlorins and bacteriochlorins |first1=Jonas |last1=Jusélius |first2=Dage |last2=Sundholm | journal = ] | year = 2000 | volume = 2 | pages = 2145–2151 | doi = 10.1039/b000260g | issue = 10|bibcode=2000PCCP....2.2145J |url=https://zenodo.org/record/900813 }}{{open access}}</ref> | ||
| ==Properties== | ==Properties, structure, bonding== | ||
| Pyrrole is a colorless ] liquid that darkens readily upon exposure to air, and is usually purified by ] immediately before use.<ref>{{cite book | title = Purification of Laboratory Chemicals | edition = 5th |last1=Armarego |first1=Wilfred L. F. |last2=Chai |first2=Christina L. L. | year = 2003 | publisher = Elsevier | pages = 346}}</ref> Pyrrole has a nutty odor. Pyrrole is a 5-membered ] heterocycle, like ] and ]. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 ]. In CDCl<sub>3</sub>, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is an extremely weak base for an amine, with a conjugate acid p''K''<sub>a</sub> of −3.8. The most thermodynamically stable pyrrolium cation (C<sub>4</sub>H<sub>6</sub>N<sup>+</sup>) is formed by protonation at the 2 position. Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid p''K''<sub>a</sub> of +3.7. Pyrrole is also weakly acidic at the N–H position, with a p''K''<sub>a</sub> of 16.5. | |||
| Pyrrole has very low basicity compared to conventional ]s and some other aromatic compounds like ]. This decreased basicity is attributed to the delocalization of the ] of ]s of the nitrogen atom in the aromatic ring. Pyrrole is a very weak base with a pK<sub>aH</sub> of about −4. Protonation results in loss of aromaticity, and is, therefore, unfavorable. | |||
| As a hydrogen bonding Lewis acid it is classified as a ] and the ] lists its acid parameters as ''E''<sub>A</sub> = 1.38 and ''C''<sub>A</sub> = 0.68. | |||
| Pyrrole has ] character because the ]s of ]s on the nitrogen atom is partially ] into the ring, creating a 4''n'' + 2 aromatic system (see ]). In terms of its aromaticity, pyrrole's is modest relative to ] but comparable to related heterocycles ] and ]. The ] of benzene, pyrrole, ], and ] are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol).<ref>{{March6th|page=62}}</ref> The molecule is flat. | |||
| Like many amines, pyrrole darkens on exposure to air and light, and needs to be distilled immediately before use.<ref>{{cite book | title = Purification of Laboratory Chemicals | edition = 5th | author = Armarego, Wilfred, L.F.; Chai, Christina, L.L. | year = 2003 | publisher = Elsevier | pages = 346}}</ref> | |||
| ==History== | |||
| <gallery> | |||
| Pyrrole was first detected by ] in 1834, as a constituent of ].<ref>{{cite journal|first=F. F. |last=Runge |date=1834 |url=http://babel.hathitrust.org/cgi/pt?id=wu.89048351654;view=1up;seq=81 |title=Ueber einige Produkte der Steinkohlendestillation |trans-title=On some products of coal distillation |journal=Annalen der Physik und Chemie |volume=31 |issue=5 |pages=65–78|doi=10.1002/andp.18341070502 |bibcode=1834AnP...107...65R }} {{open access}} See especially pages 67–68, where Runge names the compound ''Pyrrol'' (fire oil) or ''Rothöl'' (red oil).</ref> In 1857, it was isolated from the ]. Its name comes from the Greek ''pyrrhos'' ({{lang|grc|πυρρός}}, "reddish, fiery"), from the reaction used to detect it—the red color that it imparts to wood when moistened with ].<ref name="Ullmann">{{Ullmann|first=Albrecht Ludwig |last=Harreus |title=Pyrrole |doi=10.1002/14356007.a22_453}}</ref> | |||
| Image:Pyrrole Pre Distillation.jpg|Pyrrole being distilled. | |||
| Image:Pyrrole Post Distillation.jpg|Pyrrole after the ] process. It is now colorless and ] to all wavelengths of visible light due to the removal of impurities such as ]. | |||
| ==Occurrence in nature== | |||
| </gallery> | |||
| ] | |||
| Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of ] and ]. Common naturally produced molecules containing pyrroles include ], bile pigments like ] and ], and the ] of ], ], ]s, ]s, and porphyrinogens.<ref name="Jonas Jusélius and Dage Sundholm 2000 2145–2151"/> Other pyrrole-containing secondary ]s include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin. The syntheses of pyrrole-containing haemin, synthesized by ] was recognized by the Nobel Prize. | |||
| Pyrrole is a constituent of tobacco smoke and may contribute to its toxic effects.<ref>{{cite web |url=http://www.moh.govt.nz/moh.nsf/pagescm/1003/$File/chemicalconstituentscigarettespriorities.pdf |title=The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction |first1= Jefferson|last1=Fowles |first2=Michael |last2=Bates |first3=Dominique |last3=Noiton |date=March 2000 |publisher= ] |location= Porirua, New Zealand |pages=20, 49–65 |access-date=2012-09-23}}</ref> | |||
| ==Synthesis== | ==Synthesis== | ||
| Pyrrole is prepared industrially |
Pyrrole is prepared industrially by treatment of ] with ] in the presence of solid acid ]s, like ] and ].<ref name="Ullmann"/> | ||
| ] | |||
| Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine.{{cn|date=September 2023}} | |||
| One synthetic route to pyrrole involves the ] of ], the ammonium salt of ]. The salt is typically heated in a ] setup with ] as a ].<ref>Practical Organic Chemistry, Vogel, 1956, Page 837, </ref> | |||
| Several syntheses of the pyrrole ring have been described.<ref name="Lubell">{{cite journal|last1=Lubell |first1=W. |last2=Saint-Cyr |first2=D. |last3=Dufour-Gallant |first3=J. |last4=Hopewell |first4=R. |last5=Boutard |first5=N. |last6=Kassem |first6=T. |last7=Dörr |first7=A. |last8=Zelli |first8=R. |url=https://www.thieme.de/en/thieme-chemistry/sos-knowledge-updates-2013-58727.htm |title= 1''H''-Pyrroles (Update 2013) |journal=Science of Synthesis |date=2013 |volume=2013 |issue=1 |pages=157–388}}</ref> Three routes dominate,<ref name=Gil>{{cite book |last1=Gilchrist |first1=Thomas L. |title=Heterocyclic Chemistry |date=1997 |publisher=Longman |location=Liverpool |page=194-196 |edition=3rd}}</ref> but many other methods exist. | |||
| :] | |||
| ===Hantzsch pyrrole synthesis=== | |||
| {{Main|Hantzsch pyrrole synthesis}} | |||
| The Hantzsch pyrrole synthesis is the reaction of β-ketoesters ('''1''') with ammonia (or primary amines) and α-haloketones ('''2''') to give substituted pyrroles ('''3''').<ref>{{cite journal | last1 = Hantzsch | first1 = A. | year = 1890 | title = Neue Bildungsweise von Pyrrolderivaten |trans-title=New methods of forming pyrrole derivatives | url = https://babel.hathitrust.org/cgi/pt?id=uc1.b3481786;view=1up;seq=1484 | journal = Berichte der Deutschen Chemischen Gesellschaft | volume = 23 | pages = 1474–1476 | doi=10.1002/cber.189002301243}} {{open access}}</ref><ref>{{cite journal | last1 = Feist | first1 = Franz | year = 1902 | title = Studien in der Furan- und Pyrrol-Gruppe |trans-title=Studies in the furan and pyrrole groups | url = https://babel.hathitrust.org/cgi/pt?id=uiug.30112025693646;view=1up;seq=305 | journal = Berichte der Deutschen Chemischen Gesellschaft | volume = 35 | issue = 2| pages = 1537–1544 | doi = 10.1002/cber.19020350263}} {{open access}}</ref> | |||
| ] | |||
| ===Substituted pyrroles=== | |||
| Many methods exist for the ] of pyrrole derivatives. Classic "named reactions" are the ], the ], and the ]. More specialized methods are listed here. | |||
| ===Knorr pyrrole synthesis=== | |||
| The starting materials in the '''Piloty-Robinson pyrrole synthesis''' are 2 equivalents of an ] and ].<ref>{{cite journal | author = ] | journal = ] | year = 1910 | volume = 43 | pages = 489 | doi = 10.1002/cber.19100430182 | title = Synthese von Pyrrolderivaten: Pyrrole aus Succinylobernsteinsäureester, Pyrrole aus Azinen}}</ref><ref>{{cite journal | journal = ] | year = 1918 | volume = 113 | pages = 639| doi = 10.1039/CT9181300639 | title = LIV.—A new synthesis of tetraphenylpyrrole | author = Robinson, Gertrude Maud | last2 = Robinson | first2 = Robert}}</ref> The product is a pyrrole with specific substituents in the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-] (R–C=N−N=C–R), which, with added ], gives ring-closure and loss of ] to the pyrrole. | |||
| {{Main|Knorr pyrrole synthesis}} | |||
| The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound.<ref>{{Cite journal | doi = 10.1002/cber.18840170220| title = Synthese von Pyrrolderivaten |trans-title=Synthesis of pyrrole derivatives | journal = Berichte der Deutschen Chemischen Gesellschaft| volume = 17| issue = 2| pages = 1635–1642| year = 1884| last1 = Knorr | first1 = Ludwig | url = https://babel.hathitrust.org/cgi/pt?id=uc1.b3481769;view=1up;seq=65 }} {{open access}}</ref><ref>{{cite journal| last=Knorr |first=L. | title = Synthetische Versuche mit dem Acetessigester |trans-title=Synthesis experiments with the ester of acetoacetic acid | journal=Annalen der Chemie | year=1886| volume=236|issue=3 | pages= 290–332 | url = https://babel.hathitrust.org/cgi/pt?id=hvd.hx3kie;view=1up;seq=702 | doi=10.1002/jlac.18862360303}} {{open access}}</ref><ref>{{Cite journal | doi = 10.1002/cber.19020350392| title = Ueber die Bildung von Pyrrolderivaten aus Isonitrosoketonen |trans-title=On the formation of pyrrole derivatives from isonitrosketones | journal = Berichte der Deutschen Chemischen Gesellschaft| volume = 35| issue = 3| pages = 2998–3008| year = 1902| last1 = Knorr | first1 = L.| last2 = Lange | first2 = H. | url = https://babel.hathitrust.org/cgi/pt?id=uc1.b3481898;view=1up;seq=470 }} {{open access}}</ref> The method involves the reaction of an α-]] ('''1''') and a compound containing a ] α to (bonded to the next carbon to) a ] ('''2''').<ref>{{cite book|last=Corwin |first=Alsoph Henry |chapter=Chapter 6: The Chemistry of Pyrrole and its Derivatives |editor-first=Robert Cooley |editor-last=Elderfield |title=Heterocyclic Compounds |volume=1 |location=New York, NY |publisher=Wiley |date=1950 |page=287}}</ref> | |||
| ] | |||
| In one modification, ] is treated first with ] and then with ] at high temperatures and assisted by ]:<ref>{{cite journal | title = Microwave-Assisted Piloty-Robinson Synthesis of 3,4-Disubstituted Pyrroles | author = Benjamin C. Milgram, Katrine Eskildsen, Steven M. Richter, W. Robert Scheidt, and Karl A. Scheidt | journal = ] | year = 2007 | volume = 72 | issue = 10 | pages = 3941–3944 | format = Note | doi = 10.1021/jo070389 | pmid = 17432915 | pmc = 1939979}}</ref> | |||
| ===Paal–Knorr pyrrole synthesis=== | |||
| :] | |||
| {{Main|Paal–Knorr pyrrole synthesis}} | |||
| In the second step, a ] takes place between two intermediates. | |||
| In the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.<ref name="Paal1">{{citation | last=Paal|first= C.| title= Ueber die Derivate des Acetophenonacetessigesters und des Acetonylacetessigesters | journal=Berichte der Deutschen Chemischen Gesellschaft| year=1884| volume=17|issue= 2| pages=2756–2767 | url=https://babel.hathitrust.org/cgi/pt?id=uc1.b3481769;view=1up;seq=1188 |doi=10.1002/cber.188401702228}} {{open access}}</ref><ref name="Knorr1">{{citation | last=Knorr|first= Ludwig| title=Synthese von Furfuranderivaten aus dem Diacetbernsteinsäureester |trans-title=Synthesis of furan derivatives from the ester of 2,3-diacetyl-succinic acid | journal=Berichte der Deutschen Chemischen Gesellschaft| year=1884| volume=17|issue= 2| pages=2863–2870| url=https://babel.hathitrust.org/cgi/pt?id=uc1.b3481769;view=1up;seq=1295 | doi=10.1002/cber.188401702254}} {{open access}}</ref> | |||
| ] | |||
| Pyrrole can be ] to form ]. | |||
| ====Other methods==== | |||
| ==Reactivity== | |||
| ] pyrroles are produced by reaction of ] (TosMIC) with an ] in the presence of base, in a ]. A 5-''endo'' cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.{{citation needed|date=July 2016}} | |||
| The N''H'' proton in pyrroles is moderately acidic with a pK<sub>a</sub> of 16.5. Pyrrole can be ] with strong ]s such as ] and ]. The resulting alkali pyrrolide is ]. Treating this ] with an ] such as ] gives N-methylpyrrole. | |||
| :] | |||
| By the ], an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-''endo''-''dig'' cyclization, elimination of the ], and ]ization.<ref>{{cite book | first=Jie Jack |last=Li | title =Heterocyclic Chemistry in Drug Discovery | publisher =Wiley | location =New York | year =2013 | isbn =9781118354421| url =https://books.google.com/books?id=p_fM6CuK6xcC}}</ref> | |||
| ] | |||
| ] | |||
| The resonance contributors of pyrrole provide insight to the reactivity of the compound. Like ] and ], pyrrole is more reactive than benzene towards ] because it is able to stabilize the positive charge of the intermediate ]. | |||
| The starting materials in the Piloty–Robinson pyrrole synthesis, named for ] and ] and ], are two equivalents of an ] and ].<ref>{{cite journal | author-link= Oskar Piloty|last=Piloty |first=Oskar | journal = Berichte der Deutschen Chemischen Gesellschaft | year = 1910 | volume = 43 | issue = 1 | pages = 489–498 | doi = 10.1002/cber.19100430182 | title = Synthese von Pyrrolderivaten: Pyrrole aus Succinylobernsteinsäureester, Pyrrole aus Azinen |trans-title=Synthesis of pyrrole derivatives: pyrrole from diethyl succinyl succinate, pyrrole from azines | url = https://babel.hathitrust.org/cgi/pt?id=uc1.b3481273;view=1up;seq=499 }} {{open access}}</ref><ref>{{cite journal | journal = ] | year = 1918 | volume = 113 | pages = 639–645| doi = 10.1039/CT9181300639 | title = LIV. A new synthesis of tetraphenylpyrrole | last1 = Robinson|first1= Gertrude Maud | last2 = Robinson | first2 = Robert}}</ref> The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-] (R−C=N−N=C−R). In the second step, a -] takes place between. Addition of ] leads to ring closure and loss of ] to form the pyrrole. The ] was developed by the Robinsons. | |||
| Pyrrole undergoes ] predominantly at the 2 and 5 positions. Two such reactions that are especially significant for producing functionalized pyrroles are the ] and the ] (depicted below)<ref name="GP">{{cite journal | title = Comparison of Benzene, Nitrobenzene, and Dinitrobenzene 2-Arylsulfenylpyrroles | author = Jose R. Garabatos-Perera, Benjamin H. Rotstein, and Alison Thompson | journal = ] | year = 2007 | volume = 72 | pages = 7382–7385 | doi = 10.1021/jo070493r | pmid = 17705533 | issue = 19}}</ref><ref>The 2-] group in the pyrrole substrate serves as an ] and as a ] that can be removed with ] </ref> both of which are compatible with a variety of pyrrole substrates. | |||
| In one modification, ] is treated first with ] and then with ] at high temperatures and assisted by ]:<ref name=milgram>{{cite journal | title = Microwave-Assisted Piloty–Robinson Synthesis of 3,4-Disubstituted Pyrroles |first1=Benjamin C.|last1= Milgram |first2=Katrine|last2= Eskildsen |first3=Steven M.|last3= Richter |first4=W. Robert |last4=Scheidt |first5=Karl A. |last5=Scheidt | journal = ] | year = 2007 | volume = 72 | issue = 10 | pages = 3941–3944 | format = Note | doi = 10.1021/jo070389+| pmid = 17432915 | pmc = 1939979}}</ref> | |||
| :] | |||
| ] | |||
| Pyrroles react with ]s to form ]s. For example, ] condenses with pyrrole to give ]. Pyrrole compounds can also participate in ] (]) reactions under certain conditions, such as under Lewis acid catalysis, heating, or high pressure. | |||
| Pyrroles bearing multiple substituents have been obtained from the reaction of ]s and ]s. The reaction mechanism involves ] followed by loss of ] by a retro-] process. Similar reactions can be performed using azalactones. | |||
| Pyrrole polymerizes in light. Also an oxidant agent like ammonium persulfate can be use, typically at 0ºC and darkness, to control the polymerization. | |||
| ] | |||
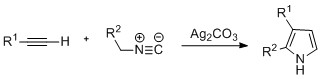
| Pyrroles can also be prepared by ]-catalyzed cyclization of alkynes with ], where R<sup>2</sup> is an electron-withdrawing group, and R<sup>1</sup> is an alkane, aryl group, or ester. Examples of disubstituted alkynes have also been seen to form the desired pyrrole in considerable yield. The reaction is proposed to proceed via a silver ] intermediate. This method is analogous to the ] ] used to form azoles. | |||
| ] | |||
| One synthetic route to pyrrole involves the ] of ], the ammonium salt of ]. The salt is typically heated in a ] setup with ] as a ].<ref>{{cite book|title=Practical Organic Chemistry |last=Vogel |date=1956 |page=837 |url=http://www.sciencemadness.org/library/books/vogel_practical_ochem_3.pdf}}</ref> | |||
| ] | |||
| ==Biosynthesis== | |||
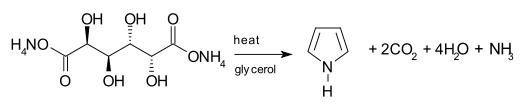
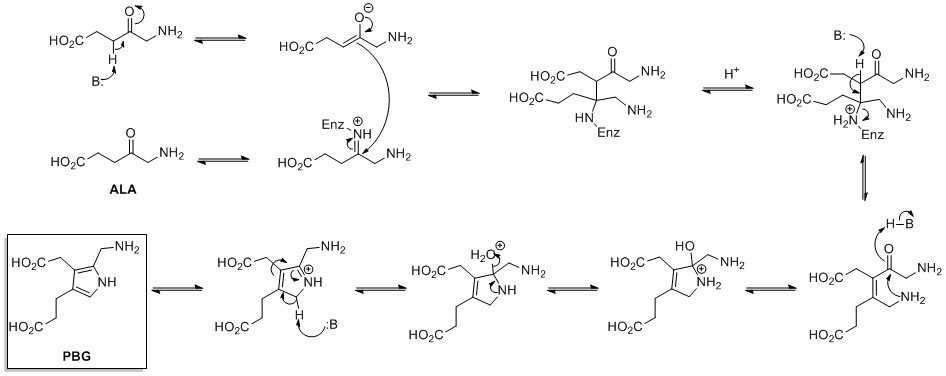
| The biosynthesis of pyrrole rings begins with ] (ALA), which is synthesized from ] and ]. ALA dehydratase catalyzes the condensation of two ALA molecules via a ] ring synthesis to form ] (PBG). This later reacts to form, for example, the macrocycles ] and ].<ref>{{cite journal|last1=Walsh|first1=Christopher T.|last2=Garneau-Tsodikova |first2=Sylvie|author2-link= Sylvie Garneau-Tsodikova |last3=Howard-Jones |first3=Annaleise R. |title=Biological formation of pyrroles: Nature's logic and enzymatic machinery|journal=Natural Product Reports|volume=23|issue=4|pages=517–531|doi=10.1039/b605245m|year=2006|pmid=16874387}}</ref> | |||
| ]. | |||
| ] is ] derived from the amino acid <small>L</small>-]. ] is first formed by ] (ATP-dependent) and ] (which requires NADH or NADPH). This can then either spontaneously cyclize to form ], which is reduced to proline by ] (using NADH or NADPH), or turned into ] by ], followed by cyclisation by ] to form proline.<ref>{{Lehninger3rd}}.</ref> | |||
| ]ic structure of both proline enantiomers: (''S'')-proline (left) and (''R'')-proline]] | |||
| Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins. ] | |||
| The biosynthesis of Prodigiosin<ref>{{cite journal | last1 = Walsh | first1 = C. T. | last2 = Garneau-Tsodikova | first2 = S. | last3 = Howard-Jones | first3 = A. R. | year = 2006 | title = Biological formation of pyrroles: Nature's logic and enzymatic machinery | journal = Nat. Prod. Rep. | volume = 23 | issue = 4 | pages = 517–531 | doi = 10.1039/b605245m | pmid = 16874387 }}</ref><ref name=Hu>{{cite journal | doi = 10.1021/acs.chemrev.6b00024 | volume=116 | title=Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products | year=2016 | journal=Chemical Reviews | pages=7818–7853 | last1 = Hu | first1 = Dennis X. | issue=14 | pmid=27314508 | pmc=5555159}}</ref> involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from <small>L</small>-proline, <small>L</small>-serine, <small>L</small>-methionine, pyruvate, and 2-octenal. | |||
| Ring A is synthesized from <small>L</small>-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD<sup>+</sup> to yield pyrrole ring A. | |||
| ] | |||
| Ring A is then expanded via the polyketide synthase pathway to incorporate <small>L</small>-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of <small>L</small>-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from <small>L</small>-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures. | |||
| ] | |||
| ==Reactions and reactivity== | |||
| Due to its ], pyrrole is difficult to ], does not easily react as a ] in ] reactions, and does not undergo usual ] reactions. Its reactivity is similar to that of ] and ], in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles ] easily to ],<ref>{{cite book|editor1=Wang Jitao |editor2=Zhang Baoshen |editor3=Wang Yongmei |editor4=Hu Qingmei |year=2003|script-title=zh:有机化学|lang=zh|trans-title=Organic Chemistry|edition=2nd|publisher=Tianjin Nankai University|isbn=978-7-310-00620-5}}</ref> and thus many ] reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including ] pyrroles) have been used in a broad range of transformations.<ref name="Lubell"/> | |||
| ===Reaction of pyrrole with electrophiles=== | |||
| Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate. ] Pyrroles react easily with ] (e.g. ]/]), ] (]), and ]ating (e.g. ], ], ], ], and ]/]) agents.<ref>{{cite encyclopedia|entry=Pyrrol|encyclopedia=Römpp Lexikon Chemie|trans-title=Römpp's Chemical Lexicon|publisher=Thieme|lang=de}}</ref> Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.{{citation needed|date=July 2016}} | |||
| ====Acylation==== | |||
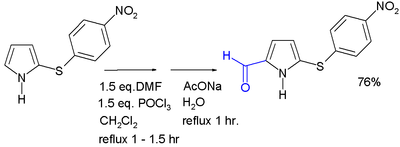
| ] generally occurs at the 2-position, through the use of various methods. Acylation with ]s and ]s can occur with or without a catalyst.<ref>{{cite journal |doi=10.15227/orgsyn.051.0100 |title=Ethyl Pyrrole-2-Carboxylate |journal=Organic Syntheses |date=1971 |volume=51 |page=100|first1=Denis M.|last1=Bailey|first2=Robert E.|last2=Johnson|first3=Noel F.|last3=Albertson }}</ref> 2-Acylpyrroles are also obtained from reaction with nitriles, by the ]. Pyrrole aldehydes can be formed by a ].<ref>{{cite journal |doi=10.15227/orgsyn.036.0074 |title=2-Pyrrolealdehyde |journal=Organic Syntheses |date=1956 |volume=36 |page=74|first1=Robert M.|last1=Silverstein|first2=Edward E.|last2=Ryskiewicz|first3=Constance|last3=Willard | |||
| }}</ref> | |||
| ] | |||
| ===Reaction of deprotonated pyrrole=== | |||
| The NH proton in pyrroles is moderately acidic with a ] of 17.5.<ref>{{cite book|first1=C. W.|last1=Bird|first2=G. W. H.|last2=Cheeseman|title=Comprehensive Heterocyclic Chemistry|url=http://www.sciencedirect.com/science/article/pii/B9780080965192000527|publisher=Pergamon|year=1984|pages=39–88|isbn=978-0-08-096519-2|postscript=None}}, although note that {{cite journal|doi=10.1016/S0040-4020(01)89212-7|journal=Tetrahedron|volume=45|issue=23|pages=7501–7504|year=1989|publisher=Pergamon|location=Great Britain|title=The acid-base properties of pyrrole and its benzologs indole and carbazole: a re-examination from the excess acidity method|first1=M.|last1=Balón|first2=M. C.|last2=Carmona|first3=M. A.|last3=Muñoz|first4=J.|last4=Hidalgo|postscript=None}} suggests that figure's revision to 17.3.</ref> Pyrrole can be deprotonated with strong bases such as ] and ].<ref>{{cite book|first1=Heinz-Gerhard|last1=Franck|first2=Jürgen Walter|last2=Stadelhofer|title=Industrielle Aromatenchemie: Rohstoffe, Verfahren, Produkte|trans-title=Industrial Chemistry of the Aromatics: Raw materials, processes, products|lang=de|publisher=Springer|location=Berlin|year=1987|isbn=978-3-662-07876-1|pages=403–404}}</ref> The resulting alkali pyrrolide is ]. Treating this conjugate base with an electrophile such as ] gives ''N''-methylpyrrole. | |||
| ''N''-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with lithium, sodium, and potassium) and more solvating solvents lead to ''N''-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom. In the cases of ''N''-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.{{citation needed|date=July 2016}} ] | |||
| Substitution at C3 can be achieved through the use of ''N''-substituted 3-bromopyrrole, which can be synthesized by bromination of ''N''-silylpyrrole with ].{{citation needed|date=July 2016}} | |||
| ===Reductions=== | |||
| Pyrroles can undergo reductions to ] and to ].<ref>{{cite book|author=Lyastukhin, Voronov |year=2006|script-title=uk:Органічна хімія|url=https://archive.org/details/isbn_9667022196|lang=uk|trans-title=Organic Chemistry|pages=781–782|isbn=966-7022-19-6}}</ref> For example, ] of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.{{citation needed|date=July 2016}} | |||
| ===Cyclization reactions=== | |||
| Pyrroles with ''N''-substitution can undergo ] reactions such as -, -, and -cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.{{citation needed|date=July 2016}} ] | |||
| Pyrroles can react with ]s, such as ], in a -cycloaddition. With ], a dichlorocyclopropane intermediate is formed, which breaks down to form ] (the Ciamician–Dennstedt rearrangement).<ref>{{cite journal|last1=Ciamician |first1=G. L. |last2=Dennstedt |first2=M. |title=Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols |trans-title=On the reaction of chloroform with the potassium compound of pyrrole |journal=Berichte der Deutschen Chemischen Gesellschaft |volume=14 |pages=1153–1162 |date=1881 |url=https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hza;view=1up;seq=1223 |doi=10.1002/cber.188101401240}}</ref><ref>{{cite book|last=Corwin |first=Alsoph Henry |editor-first=Robert Cooley |editor-last=Elderfield |title=Heterocyclic Compounds |volume=1 |location=New York, NY |publisher=Wiley |date=1950 |page=309}}</ref><ref>{{cite book|last=Mosher|first=H. S. |editor-first=Robert Cooley |editor-last=Elderfield |title=Heterocyclic Compounds |volume=1 |location=New York, NY |publisher=Wiley |date=1950 |page=475}}</ref> ] | |||
| ==Commercial uses== | ==Commercial uses== | ||
| Polypyrrole is of some commercial value. ''N''-Methylpyrrole is a precursor to ''N''-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry.<ref name="Ullmann" /> Pyrroles are also found in several drugs, including ], ], and ]. Pyrroles are used as lightfast red, scarlet, and carmine pigments.<ref>{{cite web|url=http://dyes-pigments.standardcon.com/dpp-pigments.html|title=DPP Pigments,Diketopyrrolopyrrole Pigments,DPP Pigments Wholesaler,Diketopyrrolopyrrole Pigments Suppliers|website=dyes-pigments.standardcon.com}}</ref><ref>{{cite journal|author1=Kaur, Matinder|author2=Choi, Dong Hoon|title= Diketopyrrolopyrrole: brilliant red pigment dye-based fluorescent probes and their applications|journal=Chemical Society Reviews|year=2015|pages=58–77|volume=44|issue=1|doi=10.1039/C4CS00248B|pmid=25186723}}</ref> | |||
| Pyrrole has no significant commercial application, but ''N''-methylpyrrole is a precursor to N-methylpyrrolecarboxylic acid, a building block in pharmaceutical chemistry.<ref name=Ullmann/> | |||
| ==Analogs and derivatives== | ==Analogs and derivatives== | ||
| Line 130: | Line 206: | ||
| * ], a partially saturated analog with one double bond | * ], a partially saturated analog with one double bond | ||
| * ], the saturated hydrogenated analog | * ], the saturated hydrogenated analog | ||
| ] ]s of pyrrole include: | |||
| * ], a moderately-aromatic ] analog | |||
| * ], a ] analog | |||
| * ], a ] analog | |||
| * ], an aromatic ] analog | |||
| * ], a ] analog | |||
| * ], a ] analog | |||
| * ], a non-aromatic ] analog | |||
| * ] and ], analogs with two nitrogen atoms | |||
| * ], a ] analog | |||
| * ], a ] analog | |||
| * ], an ] analog | |||
| * ], a ] analog | |||
| Derivatives of pyrrole include ], a derivative with a fused ] ring. | Derivatives of pyrrole include ], a derivative with a fused ] ring. | ||
| ==See also== | ==See also== | ||
| {{Commons category}} | |||
| * ] | |||
| * ]s | * ]s | ||
| * ] | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| ==Further reading== | |||
| * {{cite book|editor1-last=Jones|editor1-first=R. Jones|title=Pyrroles. Part I. The Synthesis and the Physical and Chemical Aspects of the Pyrrole Ring|journal=Recueil des Travaux Chimiques des Pays-Bas|series=The Chemistry of Heterocyclic Compounds|volume=48|issue=7–8|pages=351|publisher=John Wiley & Sons|location=Chichester|year=1990|isbn=978-0-471-62753-1|doi=10.1002/recl.19911100712}} | |||
| * {{cite journal | doi = 10.1016/j.tet.2006.08.071 | title = Pyrrole protection | year = 2006 | last1 = Jolicoeur | first1 = Benoit | last2 = Chapman | first2 = Erin E. | last3 = Thompson | first3 = Alison | last4 = Lubell | first4 = William D. | journal = Tetrahedron | volume = 62 | issue = 50 | pages = 11531–11563}} | |||
| ==External links== | ==External links== | ||
| * | * | ||
| * | |||
| * | |||
| * | |||
| * | |||
| {{Authority control}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 17:38, 21 February 2024
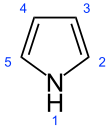
Organic ring compound (C₄H₄NH)
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-Pyrrole | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1159 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.387 | ||
| EC Number |
| ||
| Gmelin Reference | 1705 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1992, 1993 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H5N | ||
| Molar mass | 67.091 g·mol | ||
| Density | 0.967 g cm | ||
| Melting point | −23 °C (−9 °F; 250 K) | ||
| Boiling point | 129 to 131 °C (264 to 268 °F; 402 to 404 K) | ||
| Vapor pressure | 7 mmHg at 23 °C | ||
| Acidity (pKa) | 17.5 (for the N−H proton) | ||
| Basicity (pKb) | 13.6 (pKa 0.4 for C.A.) | ||
| Magnetic susceptibility (χ) | −47.6×10 cm mol | ||
| Viscosity | 0.001225 Pa s | ||
| Thermochemistry | |||
| Heat capacity (C) | 1.903 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
108.2 kJ mol (gas) | ||
| Std enthalpy of combustion (ΔcH298) |
2242 kJ mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 33.33 °C (91.99 °F; 306.48 K) | ||
| Autoignition temperature |
550 °C (1,022 °F; 823 K) | ||
| Explosive limits | 3.1–14.8% | ||
| Safety data sheet (SDS) | Chemical Safety Data | ||
| Related compounds | |||
| Related compounds | Phosphole, arsole, bismole, stibole | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls.
Properties, structure, bonding
Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is an extremely weak base for an amine, with a conjugate acid pKa of −3.8. The most thermodynamically stable pyrrolium cation (C4H6N) is formed by protonation at the 2 position. Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid pKa of +3.7. Pyrrole is also weakly acidic at the N–H position, with a pKa of 16.5. As a hydrogen bonding Lewis acid it is classified as a hard acid and the ECW model lists its acid parameters as EA = 1.38 and CA = 0.68.
Pyrrole has aromatic character because the lone pairs of electrons on the nitrogen atom is partially delocalized into the ring, creating a 4n + 2 aromatic system (see Hückel's rule). In terms of its aromaticity, pyrrole's is modest relative to benzene but comparable to related heterocycles thiophene and furan. The resonance energies of benzene, pyrrole, thiophene, and furan are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol). The molecule is flat.
History
Pyrrole was first detected by F. F. Runge in 1834, as a constituent of coal tar. In 1857, it was isolated from the pyrolysate of bone. Its name comes from the Greek pyrrhos (πυρρός, "reddish, fiery"), from the reaction used to detect it—the red color that it imparts to wood when moistened with hydrochloric acid.
Occurrence in nature

Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of cofactors and natural products. Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin and biliverdin, and the porphyrins of heme, chlorophyll, chlorins, bacteriochlorins, and porphyrinogens. Other pyrrole-containing secondary metabolites include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin. The syntheses of pyrrole-containing haemin, synthesized by Hans Fischer was recognized by the Nobel Prize.
Pyrrole is a constituent of tobacco smoke and may contribute to its toxic effects.
Synthesis
Pyrrole is prepared industrially by treatment of furan with ammonia in the presence of solid acid catalysts, like SiO2 and Al2O3.

Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine.
Several syntheses of the pyrrole ring have been described. Three routes dominate, but many other methods exist.
Hantzsch pyrrole synthesis
Main article: Hantzsch pyrrole synthesisThe Hantzsch pyrrole synthesis is the reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).

Knorr pyrrole synthesis
Main article: Knorr pyrrole synthesisThe Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound. The method involves the reaction of an α-aminoketone (1) and a compound containing a methylene group α to (bonded to the next carbon to) a carbonyl group (2).

Paal–Knorr pyrrole synthesis
Main article: Paal–Knorr pyrrole synthesisIn the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.

Other methods
Van Leusen reaction pyrroles are produced by reaction of tosylmethyl isocyanide (TosMIC) with an enone in the presence of base, in a Michael addition. A 5-endo cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.
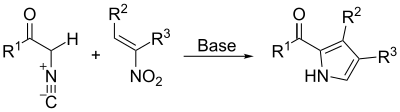
By the Barton–Zard synthesis, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-endo-dig cyclization, elimination of the nitro group, and tautomerization.
The starting materials in the Piloty–Robinson pyrrole synthesis, named for Gertrude and Robert Robinson and Oskar Piloty, are two equivalents of an aldehyde and hydrazine. The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-imine (R−C=N−N=C−R). In the second step, a -sigmatropic rearrangement takes place between. Addition of hydrochloric acid leads to ring closure and loss of ammonia to form the pyrrole. The mechanism was developed by the Robinsons.
In one modification, propionaldehyde is treated first with hydrazine and then with benzoyl chloride at high temperatures and assisted by microwave irradiation:

Pyrroles bearing multiple substituents have been obtained from the reaction of münchnones and alkynes. The reaction mechanism involves 1,3-dipolar cycloaddition followed by loss of carbon dioxide by a retro-Diels–Alder process. Similar reactions can be performed using azalactones.

Pyrroles can also be prepared by silver-catalyzed cyclization of alkynes with isonitriles, where R is an electron-withdrawing group, and R is an alkane, aryl group, or ester. Examples of disubstituted alkynes have also been seen to form the desired pyrrole in considerable yield. The reaction is proposed to proceed via a silver acetylide intermediate. This method is analogous to the azide–alkyne click chemistry used to form azoles.
One synthetic route to pyrrole involves the decarboxylation of ammonium mucate, the ammonium salt of mucic acid. The salt is typically heated in a distillation setup with glycerol as a solvent.
Biosynthesis
The biosynthesis of pyrrole rings begins with aminolevulinic acid (ALA), which is synthesized from glycine and succinyl-CoA. ALA dehydratase catalyzes the condensation of two ALA molecules via a Knorr-type ring synthesis to form porphobilinogen (PBG). This later reacts to form, for example, the macrocycles heme and chlorophyll.
.
Proline is biosynthetically derived from the amino acid L-glutamate. Glutamate-5-semialdehyde is first formed by glutamate 5-kinase (ATP-dependent) and glutamate-5-semialdehyde dehydrogenase (which requires NADH or NADPH). This can then either spontaneously cyclize to form 1-pyrroline-5-carboxylic acid, which is reduced to proline by pyrroline-5-carboxylate reductase (using NADH or NADPH), or turned into ornithine by ornithine aminotransferase, followed by cyclisation by ornithine cyclodeaminase to form proline.

Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.

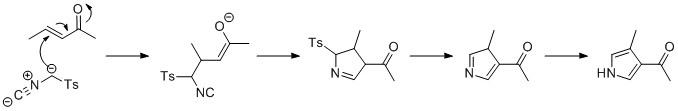
The biosynthesis of Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from L-proline, L-serine, L-methionine, pyruvate, and 2-octenal.
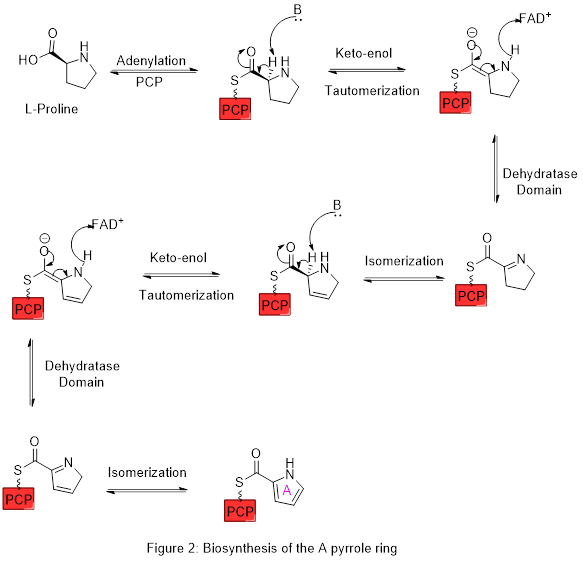
Ring A is synthesized from L-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD to yield pyrrole ring A.
Ring A is then expanded via the polyketide synthase pathway to incorporate L-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of L-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from L-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
Reactions and reactivity
Due to its aromatic character, pyrrole is difficult to hydrogenate, does not easily react as a diene in Diels–Alder reactions, and does not undergo usual olefin reactions. Its reactivity is similar to that of benzene and aniline, in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles oxidize easily to polypyrrole, and thus many electrophilic reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including protected pyrroles) have been used in a broad range of transformations.
Reaction of pyrrole with electrophiles
Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate.

Pyrroles react easily with nitrating (e.g. HNO3/Ac2O), sulfonating (Py·SO3), and halogenating (e.g. NCS, NBS, Br2, SO2Cl2, and KI/H2O2) agents. Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.
Acylation
Acylation generally occurs at the 2-position, through the use of various methods. Acylation with acid anhydrides and acid chlorides can occur with or without a catalyst. 2-Acylpyrroles are also obtained from reaction with nitriles, by the Houben–Hoesch reaction. Pyrrole aldehydes can be formed by a Vilsmeier–Haack reaction.
Reaction of deprotonated pyrrole
The NH proton in pyrroles is moderately acidic with a pKa of 17.5. Pyrrole can be deprotonated with strong bases such as butyllithium and sodium hydride. The resulting alkali pyrrolide is nucleophilic. Treating this conjugate base with an electrophile such as iodomethane gives N-methylpyrrole.
N-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with lithium, sodium, and potassium) and more solvating solvents lead to N-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom. In the cases of N-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.
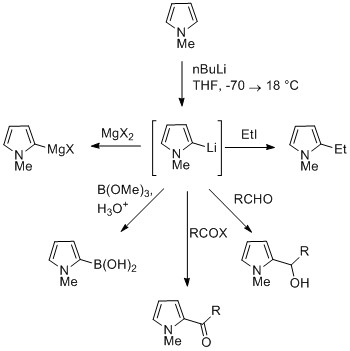
Substitution at C3 can be achieved through the use of N-substituted 3-bromopyrrole, which can be synthesized by bromination of N-silylpyrrole with NBS.
Reductions
Pyrroles can undergo reductions to pyrrolidines and to pyrrolines. For example, Birch reduction of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
Cyclization reactions
Pyrroles with N-substitution can undergo cycloaddition reactions such as -, -, and -cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.
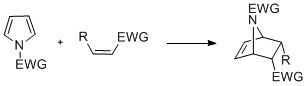
Pyrroles can react with carbenes, such as dichlorocarbene, in a -cycloaddition. With dichlorocarbene, a dichlorocyclopropane intermediate is formed, which breaks down to form 3-chloropyridine (the Ciamician–Dennstedt rearrangement).
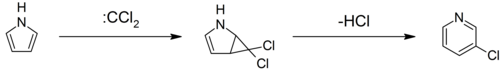
Commercial uses
Polypyrrole is of some commercial value. N-Methylpyrrole is a precursor to N-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Pyrroles are also found in several drugs, including atorvastatin, ketorolac, and sunitinib. Pyrroles are used as lightfast red, scarlet, and carmine pigments.
Analogs and derivatives
Structural analogs of pyrrole include:
- Pyrroline, a partially saturated analog with one double bond
- Pyrrolidine, the saturated hydrogenated analog
Derivatives of pyrrole include indole, a derivative with a fused benzene ring.
See also
References
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–478. ISBN 978-1-4987-5429-3.
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 141. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Loudon, Marc G. (2002). "Chemistry of Naphthalene and the Aromatic Heterocycles". Organic Chemistry (4th ed.). New York: Oxford University Press. pp. 1135–1136. ISBN 978-0-19-511999-2.
- Cox, Michael; Lehninger, Albert L.; Nelson, David R. (2000). Lehninger Principles of Biochemistry. New York: Worth Publishers. ISBN 978-1-57259-153-0.
- ^ Jusélius, Jonas; Sundholm, Dage (2000). "The aromatic pathways of porphins, chlorins and bacteriochlorins". Phys. Chem. Chem. Phys. 2 (10): 2145–2151. Bibcode:2000PCCP....2.2145J. doi:10.1039/b000260g.

- Armarego, Wilfred L. F.; Chai, Christina L. L. (2003). Purification of Laboratory Chemicals (5th ed.). Elsevier. p. 346.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 62, ISBN 978-0-471-72091-1
- Runge, F. F. (1834). "Ueber einige Produkte der Steinkohlendestillation" [On some products of coal distillation]. Annalen der Physik und Chemie. 31 (5): 65–78. Bibcode:1834AnP...107...65R. doi:10.1002/andp.18341070502.
 See especially pages 67–68, where Runge names the compound Pyrrol (fire oil) or Rothöl (red oil).
See especially pages 67–68, where Runge names the compound Pyrrol (fire oil) or Rothöl (red oil).
- ^ Harreus, Albrecht Ludwig. "Pyrrole". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_453. ISBN 978-3527306732.
- Fowles, Jefferson; Bates, Michael; Noiton, Dominique (March 2000). "The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction" (PDF). Porirua, New Zealand: New Zealand Ministry of Health. pp. 20, 49–65. Retrieved 2012-09-23.
- ^ Lubell, W.; Saint-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R. (2013). "1H-Pyrroles (Update 2013)". Science of Synthesis. 2013 (1): 157–388.
- Gilchrist, Thomas L. (1997). Heterocyclic Chemistry (3rd ed.). Liverpool: Longman. p. 194-196.
- Hantzsch, A. (1890). "Neue Bildungsweise von Pyrrolderivaten" [New methods of forming pyrrole derivatives]. Berichte der Deutschen Chemischen Gesellschaft. 23: 1474–1476. doi:10.1002/cber.189002301243.

- Feist, Franz (1902). "Studien in der Furan- und Pyrrol-Gruppe" [Studies in the furan and pyrrole groups]. Berichte der Deutschen Chemischen Gesellschaft. 35 (2): 1537–1544. doi:10.1002/cber.19020350263.

- Knorr, Ludwig (1884). "Synthese von Pyrrolderivaten" [Synthesis of pyrrole derivatives]. Berichte der Deutschen Chemischen Gesellschaft. 17 (2): 1635–1642. doi:10.1002/cber.18840170220.

- Knorr, L. (1886). "Synthetische Versuche mit dem Acetessigester" [Synthesis experiments with the ester of acetoacetic acid]. Annalen der Chemie. 236 (3): 290–332. doi:10.1002/jlac.18862360303.

- Knorr, L.; Lange, H. (1902). "Ueber die Bildung von Pyrrolderivaten aus Isonitrosoketonen" [On the formation of pyrrole derivatives from isonitrosketones]. Berichte der Deutschen Chemischen Gesellschaft. 35 (3): 2998–3008. doi:10.1002/cber.19020350392.

- Corwin, Alsoph Henry (1950). "Chapter 6: The Chemistry of Pyrrole and its Derivatives". In Elderfield, Robert Cooley (ed.). Heterocyclic Compounds. Vol. 1. New York, NY: Wiley. p. 287.
- Paal, C. (1884), "Ueber die Derivate des Acetophenonacetessigesters und des Acetonylacetessigesters", Berichte der Deutschen Chemischen Gesellschaft, 17 (2): 2756–2767, doi:10.1002/cber.188401702228

- Knorr, Ludwig (1884), "Synthese von Furfuranderivaten aus dem Diacetbernsteinsäureester" [Synthesis of furan derivatives from the ester of 2,3-diacetyl-succinic acid], Berichte der Deutschen Chemischen Gesellschaft, 17 (2): 2863–2870, doi:10.1002/cber.188401702254

- Li, Jie Jack (2013). Heterocyclic Chemistry in Drug Discovery. New York: Wiley. ISBN 9781118354421.
- Piloty, Oskar (1910). "Synthese von Pyrrolderivaten: Pyrrole aus Succinylobernsteinsäureester, Pyrrole aus Azinen" [Synthesis of pyrrole derivatives: pyrrole from diethyl succinyl succinate, pyrrole from azines]. Berichte der Deutschen Chemischen Gesellschaft. 43 (1): 489–498. doi:10.1002/cber.19100430182.

- Robinson, Gertrude Maud; Robinson, Robert (1918). "LIV. A new synthesis of tetraphenylpyrrole". J. Chem. Soc. 113: 639–645. doi:10.1039/CT9181300639.
- ^ Milgram, Benjamin C.; Eskildsen, Katrine; Richter, Steven M.; Scheidt, W. Robert; Scheidt, Karl A. (2007). "Microwave-Assisted Piloty–Robinson Synthesis of 3,4-Disubstituted Pyrroles" (Note). J. Org. Chem. 72 (10): 3941–3944. doi:10.1021/jo070389+. PMC 1939979. PMID 17432915.
- Vogel (1956). Practical Organic Chemistry (PDF). p. 837.
- Walsh, Christopher T.; Garneau-Tsodikova, Sylvie; Howard-Jones, Annaleise R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery". Natural Product Reports. 23 (4): 517–531. doi:10.1039/b605245m. PMID 16874387.
- Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000). Principles of Biochemistry (3rd ed.). New York: W. H. Freeman. ISBN 1-57259-153-6..
- Walsh, C. T.; Garneau-Tsodikova, S.; Howard-Jones, A. R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery". Nat. Prod. Rep. 23 (4): 517–531. doi:10.1039/b605245m. PMID 16874387.
- Hu, Dennis X. (2016). "Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products". Chemical Reviews. 116 (14): 7818–7853. doi:10.1021/acs.chemrev.6b00024. PMC 5555159. PMID 27314508.
- Wang Jitao ; Zhang Baoshen ; Wang Yongmei ; Hu Qingmei , eds. (2003). 有机化学 [Organic Chemistry] (in Chinese) (2nd ed.). Tianjin Nankai University. ISBN 978-7-310-00620-5.
- "Pyrrol". Römpp Lexikon Chemie (in German). Thieme.
- Bailey, Denis M.; Johnson, Robert E.; Albertson, Noel F. (1971). "Ethyl Pyrrole-2-Carboxylate". Organic Syntheses. 51: 100. doi:10.15227/orgsyn.051.0100.
- Silverstein, Robert M.; Ryskiewicz, Edward E.; Willard, Constance (1956). "2-Pyrrolealdehyde". Organic Syntheses. 36: 74. doi:10.15227/orgsyn.036.0074.
- Bird, C. W.; Cheeseman, G. W. H. (1984). Comprehensive Heterocyclic Chemistry. Pergamon. pp. 39–88. ISBN 978-0-08-096519-2, although note that Balón, M.; Carmona, M. C.; Muñoz, M. A.; Hidalgo, J. (1989). "The acid-base properties of pyrrole and its benzologs indole and carbazole: a re-examination from the excess acidity method". Tetrahedron. 45 (23). Great Britain: Pergamon: 7501–7504. doi:10.1016/S0040-4020(01)89212-7 suggests that figure's revision to 17.3.
- Franck, Heinz-Gerhard; Stadelhofer, Jürgen Walter (1987). Industrielle Aromatenchemie: Rohstoffe, Verfahren, Produkte [Industrial Chemistry of the Aromatics: Raw materials, processes, products] (in German). Berlin: Springer. pp. 403–404. ISBN 978-3-662-07876-1.
- Lyastukhin, Voronov (2006). Органічна хімія [Organic Chemistry] (in Ukrainian). pp. 781–782. ISBN 966-7022-19-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Ciamician, G. L.; Dennstedt, M. (1881). "Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols" [On the reaction of chloroform with the potassium compound of pyrrole]. Berichte der Deutschen Chemischen Gesellschaft. 14: 1153–1162. doi:10.1002/cber.188101401240.
- Corwin, Alsoph Henry (1950). Elderfield, Robert Cooley (ed.). Heterocyclic Compounds. Vol. 1. New York, NY: Wiley. p. 309.
- Mosher, H. S. (1950). Elderfield, Robert Cooley (ed.). Heterocyclic Compounds. Vol. 1. New York, NY: Wiley. p. 475.
- "DPP Pigments,Diketopyrrolopyrrole Pigments,DPP Pigments Wholesaler,Diketopyrrolopyrrole Pigments Suppliers". dyes-pigments.standardcon.com.
- Kaur, Matinder; Choi, Dong Hoon (2015). "Diketopyrrolopyrrole: brilliant red pigment dye-based fluorescent probes and their applications". Chemical Society Reviews. 44 (1): 58–77. doi:10.1039/C4CS00248B. PMID 25186723.
Further reading
- Jones, R. Jones, ed. (1990). Pyrroles. Part I. The Synthesis and the Physical and Chemical Aspects of the Pyrrole Ring. The Chemistry of Heterocyclic Compounds. Vol. 48. Chichester: John Wiley & Sons. p. 351. doi:10.1002/recl.19911100712. ISBN 978-0-471-62753-1.
{{cite book}}:|journal=ignored (help) - Jolicoeur, Benoit; Chapman, Erin E.; Thompson, Alison; Lubell, William D. (2006). "Pyrrole protection". Tetrahedron. 62 (50): 11531–11563. doi:10.1016/j.tet.2006.08.071.
External links
- Synthesis of pyrroles (overview of recent methods)
- Substitution reaction mechanisms of nitrogen-containing heteroaromatics