| Revision as of 10:39, 5 April 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: PubChem.← Previous edit | Latest revision as of 10:16, 13 July 2023 edit undoIndian but not spicy (talk | contribs)223 edits →CompoundsTag: Visual edit | ||
| (52 intermediate revisions by 32 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = 444334205 | |||
| | Verifiedfields = changed | |||
| | ImageFile = Biopterin.svg | |||
| | Watchedfields = changed | |||
| | ImageSize = 230 | |||
| | verifiedrevid = 399692725 | |||
| | ImageAlt = Skeletal formula of biopterin | |||
| |ImageFile=Biopterin.svg | |||
| | ImageFile1 = Biopterin-3D-balls.png | |||
| |ImageSize= | |||
| | ImageSize1 = 230 | |||
| |IUPACName= 2-Amino-6-(1,2-dihydroxypropyl)-1''H''-pteridin-4-one | |||
| | ImageAlt1 = Ball-and-stick model of the biopterin molecule (tautomer CID 445040) | |||
| |OtherNames= | |||
| | IUPACName= 2-Amino-6-(1,2-dihydroxypropyl)-1''H''-pteridin-4-one | |||
| |Section1= {{Chembox Identifiers | |||
| | OtherNames= | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |Section1={{Chembox Identifiers | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 392795 | | ChemSpiderID = 392795 | ||
| | KEGG_Ref = {{keggcite| |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C06313 | | KEGG = C06313 | ||
| | InChI = 1/C9H11N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h2-3,6,15-16H,1H3,(H3,10,11,13,14,17) | | InChI = 1/C9H11N5O3/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7/h2-3,6,15-16H,1H3,(H3,10,11,13,14,17) | ||
| Line 21: | Line 23: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = LHQIJBMDNUYRAM-DZSWIPIPSA-N | | StdInChIKey = LHQIJBMDNUYRAM-DZSWIPIPSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo=22150-76-1 | | CASNo=22150-76-1 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | PubChem = 445040 | |||
| | UNII = E54CTM794Y | |||
| | SMILES = O=C2\N=C(/Nc1ncc(nc12)C(O)C(O)C)N | |||
| | PubChem = 445040 | |||
| | MeSHName=Biopterin | |||
| | SMILES = O=C2\N=C(/Nc1ncc(nc12)C(O)C(O)C)N | |||
| | MeSHName=Biopterin | |||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | |
| Formula=C<sub>9</sub>H<sub>11</sub>N<sub>5</sub>O<sub>3</sub> | ||
| | |
| MolarMass=237.216 g/mol | ||
| | |
| Appearance= | ||
| | |
| Density= | ||
| | |
| MeltingPt= | ||
| | |
| BoilingPt= | ||
| | |
| Solubility= | ||
| }} | }} | ||
| |Section3= |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards= | ||
| | |
| FlashPt= | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| }} | }} | ||
| }} | }} | ||
| '''Biopterins''' are ] derivatives which function as endogenous enzyme ]s in many species of animals and in some bacteria and fungi. The prototypical compound of the class is '''biopterin''' (6-(1,2-dihydroxypropyl)-pterin), as shown in the infobox. Biopterins act as cofactors for ] (AAAH), which are involved in synthesizing a number of ]s including ], ], ], and ], along with several ]s. ] synthesis also uses biopterin derivatives as cofactors. In humans, ] (BH4) is the endogenous cofactor for AAAH enzymes. | |||
| '''Biopterin''' is a ] that is produced within the body. | |||
| As with pterins in general, biopterins exhibit ]. In other words, there are a number of forms that readily interconvert, differing by the placement of hydrogen atoms. Depictions of the chemical structure may therefore vary among sources.<ref>{{cite journal |last1=Benkovic |first1=Stephen J. |last2=Sammons |first2=Douglas |last3=Armarego |first3=Wilfred L. F. |last4=Waring |first4=Paul |last5=Inners |first5=Ruth |title=Tautomeric nature of quinonoid 6,7-dimethyl-7,8-dihydro-6H-pterin in aqueous solution: a nitrogen-15 NMR study |journal=Journal of the American Chemical Society |date=June 1985 |volume=107 |issue=12 |pages=3706–3712 |doi=10.1021/ja00298a048}}</ref> | |||
| It is an oxidized degradation product of ]. | |||
| == Compounds == | |||
| Defects in biopterin synthesis or regeneration can cause a form of ] (a disease with symptoms similar to ]) <ref></ref>. | |||
| Biopterin compounds found in the animal body include ], the ]<ref>{{cite journal |doi=10.1074/jbc.M302227200 |pmid=12692136 |title=Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols: Implications For Uncoupling Endothelial Nitric-Oxide Synthase |journal=Journal of Biological Chemistry |volume=278 |issue=25 |pages=22546–54 |year=2003 |last1=Kuzkaya |first1=N. |last2=Weissmann |first2=N. |last3=Harrison |first3=D. G. |last4=Dikalov |first4=S. |doi-access=free }}</ref> BH3•, and the semi-oxidized form ]. The fully oxidized form, i.e. "biopterin" proper, has little biological significance. | |||
| Bacteria produce several unique ]s of biopterin (and of other pterins as well), using a specific BPt glucosyltransferase. They may have a function in ] protection.<ref>{{cite journal |last1=Feirer |first1=Nathan |last2=Fuqua |first2=Clay |title=Pterin function in bacteria |journal=Pteridines |date=1 May 2017 |volume=28 |issue=1 |pages=23–36 |doi=10.1515/pterid-2016-0012|s2cid=91132135 |url=http://iu.tind.io/record/732/files/0448_Pterin-function-in-bacteria.pdf }}</ref> | |||
| Biopterin is synthesized in several parts of the body, including the pineal gland<ref></ref>. | |||
| == Biosynthesis == | |||
| BH4 is the principal active cofactor. BH4 synthesis occurs through two principal pathways; the de novo pathway involves three enzymatic steps and proceeds from ], while the salvage pathway converts ] to BH4 using ].<ref>{{Cite journal | |||
| | pmid = 6572916 | |||
| | pmc = 393638 | |||
| | year = 1983 | |||
| | last1 = Nichol | |||
| | first1 = C. A. | |||
| | title = Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo | |||
| | journal = Proceedings of the National Academy of Sciences of the United States of America | |||
| | volume = 80 | |||
| | issue = 6 | |||
| | pages = 1546–50 | |||
| | last2 = Lee | |||
| | first2 = C. L. | |||
| | last3 = Edelstein | |||
| | first3 = M. P. | |||
| | last4 = Chao | |||
| | first4 = J. Y. | |||
| | last5 = Duch | |||
| | first5 = D. S. | |||
| | doi=10.1073/pnas.80.6.1546 | |||
| | bibcode = 1983PNAS...80.1546N | |||
| | doi-access = free | |||
| }}</ref> In addition, BH2 is recycled to BH4 by ]. | |||
| In the ''de novo'' pathway, GTP is converted to 7,8-dihydro] triphosphate by ] (GTPCH-1, ''FolE''), which expands the imidazole ring in GTP by one carbon. The intermediate is converted to 6-pyruvoyl-5,6,7,8-tetrahydropterin by ], which removes the phosphate and produces the diketone (pyruvoyl) substituent. The final stage is mediated by ].<ref>{{cite journal|doi=10.1042/BJ20110293|title=Tetrahydrobiopterin: Biochemistry and pathophysiology |year=2011 |last1=Werner |first1=Ernst R. |last2=Blau |first2=Nenad |last3=Thöny |first3=Beat |journal=Biochemical Journal |volume=438 |issue=3 |pages=397–414 |pmid=21867484 }}</ref> | |||
| ] | |||
| == Biopterin disorders == | |||
| {{main|Tetrahydrobiopterin deficiency|Tetrahydrobiopterin-deficient hyperphenylalaninemia}} | |||
| A number of disorders of biopterin regulation exist. | |||
| Single-gene defects affecting the gene ] block the first step in biopterin synthesis, and lead to ], also known as Segawa's syndrome. This is due to the role of BH4 in synthesising neurotransmitters, including Dopamine, and is treated with supplementation with ], which does not require BH4 for conversion to dopamine. GCH1 defects are autosomal dominant, meaning that only one defective gene copy is required for the condition to occur. Mouse gene knockout models that block biopterin synthesis completely die shortly after birth due to their inability to produce catecholamines and neurotransmitters.<ref>{{Cite journal | |||
| | pmid = 12734191 | |||
| | year = 2003 | |||
| | last1 = Elzaouk | |||
| | first1 = L | |||
| | title = Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts-/- mice rescued by feeding neurotransmitter precursors and H4-biopterin | |||
| | journal = Journal of Biological Chemistry | |||
| | volume = 278 | |||
| | issue = 30 | |||
| | pages = 28303–11 | |||
| | last2 = Leimbacher | |||
| | first2 = W | |||
| | last3 = Turri | |||
| | first3 = M | |||
| | last4 = Ledermann | |||
| | first4 = B | |||
| | last5 = Burki | |||
| | first5 = K | |||
| | last6 = Blau | |||
| | first6 = N | |||
| | last7 = Thony | |||
| | first7 = B | |||
| | doi = 10.1074/jbc.M303986200 | |||
| | doi-access = free | |||
| }}</ref> | |||
| Biopterin synthesis disorders are also a cause of hyperphenylalaninemia; phenylalanine metabolism requires BH4 as a cofactor.<ref>{{cite web |url=http://www.uic.edu/classes/phar/phar332/Clinical_Cases/aa%20metab%20cases/PKU%20Cases/bioh4.htm |title=Tetrahydrobiopterin |accessdate=2007-06-07 |url-status=dead |archiveurl=https://web.archive.org/web/20070629030636/http://www.uic.edu/classes/phar/phar332/Clinical_Cases/aa%20metab%20cases/PKU%20Cases/bioh4.htm |archivedate=2007-06-29 }}</ref> | |||
| In psychiatry, imbalances of biopterin concentrations have been hypothesized to be linked to mood disorders, particularly depression.<ref> Cavaleri et al. Blood concentrations of neopterin and biopterin in subjects with depression: A systematic review and meta-analysis ''Progress in Neuro-Psychopharmacology and Biological Psychiatry'' 2022. http://dx.doi.org/10.1016/j.pnpbp.2022.110633 </ref> | |||
| Biopterin deficiency has been associated with a variety of disorders, including ] <ref></ref> and ]<ref name="SciAmApr07">Rodney E. Willoughby, Jr., "A Cure for Rabies?" ''Scientific American'', V. 256, No. 4, April 2007, p. 95 ()</ref>. | |||
| ==References== | ==References== | ||
| {{Reflist}} | |||
| <references /> | |||
| ==External links== | |||
| * | |||
| WiseGeek. (2012). What is biopterin?. Retrieved from http://www.wisegeek.com/what-is-biopterin.htm | |||
| ] | ] | ||
| ] | ] | ||
| {{biochem-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 10:16, 13 July 2023

| |
| |
| Names | |
|---|---|
| IUPAC name 2-Amino-6-(1,2-dihydroxypropyl)-1H-pteridin-4-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.040.719 |
| KEGG | |
| MeSH | Biopterin |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H11N5O3 |
| Molar mass | 237.216 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
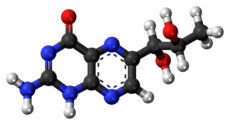
Biopterins are pterin derivatives which function as endogenous enzyme cofactors in many species of animals and in some bacteria and fungi. The prototypical compound of the class is biopterin (6-(1,2-dihydroxypropyl)-pterin), as shown in the infobox. Biopterins act as cofactors for aromatic amino acid hydroxylases (AAAH), which are involved in synthesizing a number of neurotransmitters including dopamine, norepinephrine, epinepherine, and serotonin, along with several trace amines. Nitric oxide synthesis also uses biopterin derivatives as cofactors. In humans, tetrahydrobiopterin (BH4) is the endogenous cofactor for AAAH enzymes.
As with pterins in general, biopterins exhibit tautomerism. In other words, there are a number of forms that readily interconvert, differing by the placement of hydrogen atoms. Depictions of the chemical structure may therefore vary among sources.
Compounds
Biopterin compounds found in the animal body include BH4, the free radical BH3•, and the semi-oxidized form BH2. The fully oxidized form, i.e. "biopterin" proper, has little biological significance.
Bacteria produce several unique glycosides of biopterin (and of other pterins as well), using a specific BPt glucosyltransferase. They may have a function in UV protection.
Biosynthesis
BH4 is the principal active cofactor. BH4 synthesis occurs through two principal pathways; the de novo pathway involves three enzymatic steps and proceeds from GTP, while the salvage pathway converts sepiapterin to BH4 using dihydrofolate reductase. In addition, BH2 is recycled to BH4 by dihydrobiopterin reductase.
In the de novo pathway, GTP is converted to 7,8-dihydroneopterin triphosphate by GTP cyclohydrolase I (GTPCH-1, FolE), which expands the imidazole ring in GTP by one carbon. The intermediate is converted to 6-pyruvoyl-5,6,7,8-tetrahydropterin by 6-pyruvoyltetrahydropterin synthase, which removes the phosphate and produces the diketone (pyruvoyl) substituent. The final stage is mediated by sepiapterin reductase.

Biopterin disorders
Main articles: Tetrahydrobiopterin deficiency and Tetrahydrobiopterin-deficient hyperphenylalaninemiaA number of disorders of biopterin regulation exist.
Single-gene defects affecting the gene GCH1 block the first step in biopterin synthesis, and lead to dopamine-responsive dystonia, also known as Segawa's syndrome. This is due to the role of BH4 in synthesising neurotransmitters, including Dopamine, and is treated with supplementation with levodopa, which does not require BH4 for conversion to dopamine. GCH1 defects are autosomal dominant, meaning that only one defective gene copy is required for the condition to occur. Mouse gene knockout models that block biopterin synthesis completely die shortly after birth due to their inability to produce catecholamines and neurotransmitters.
Biopterin synthesis disorders are also a cause of hyperphenylalaninemia; phenylalanine metabolism requires BH4 as a cofactor.
In psychiatry, imbalances of biopterin concentrations have been hypothesized to be linked to mood disorders, particularly depression.
References
- Benkovic, Stephen J.; Sammons, Douglas; Armarego, Wilfred L. F.; Waring, Paul; Inners, Ruth (June 1985). "Tautomeric nature of quinonoid 6,7-dimethyl-7,8-dihydro-6H-pterin in aqueous solution: a nitrogen-15 NMR study". Journal of the American Chemical Society. 107 (12): 3706–3712. doi:10.1021/ja00298a048.
- Kuzkaya, N.; Weissmann, N.; Harrison, D. G.; Dikalov, S. (2003). "Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols: Implications For Uncoupling Endothelial Nitric-Oxide Synthase". Journal of Biological Chemistry. 278 (25): 22546–54. doi:10.1074/jbc.M302227200. PMID 12692136.
- Feirer, Nathan; Fuqua, Clay (1 May 2017). "Pterin function in bacteria" (PDF). Pteridines. 28 (1): 23–36. doi:10.1515/pterid-2016-0012. S2CID 91132135.
- Nichol, C. A.; Lee, C. L.; Edelstein, M. P.; Chao, J. Y.; Duch, D. S. (1983). "Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo". Proceedings of the National Academy of Sciences of the United States of America. 80 (6): 1546–50. Bibcode:1983PNAS...80.1546N. doi:10.1073/pnas.80.6.1546. PMC 393638. PMID 6572916.
- Werner, Ernst R.; Blau, Nenad; Thöny, Beat (2011). "Tetrahydrobiopterin: Biochemistry and pathophysiology". Biochemical Journal. 438 (3): 397–414. doi:10.1042/BJ20110293. PMID 21867484.
- Elzaouk, L; Leimbacher, W; Turri, M; Ledermann, B; Burki, K; Blau, N; Thony, B (2003). "Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts-/- mice rescued by feeding neurotransmitter precursors and H4-biopterin". Journal of Biological Chemistry. 278 (30): 28303–11. doi:10.1074/jbc.M303986200. PMID 12734191.
- "Tetrahydrobiopterin". Archived from the original on 2007-06-29. Retrieved 2007-06-07.
- Cavaleri et al. Blood concentrations of neopterin and biopterin in subjects with depression: A systematic review and meta-analysis Progress in Neuro-Psychopharmacology and Biological Psychiatry 2022. http://dx.doi.org/10.1016/j.pnpbp.2022.110633
External links
WiseGeek. (2012). What is biopterin?. Retrieved from http://www.wisegeek.com/what-is-biopterin.htm
Categories: