| Revision as of 14:07, 21 April 2011 editKupirijo (talk | contribs)Extended confirmed users9,131 editsm →Function in protein synthesis: However, transformylase← Previous edit | Latest revision as of 21:01, 6 November 2024 edit undoBvos9 (talk | contribs)2 editsm →Function in protein synthesis: spelling mistakeTag: Visual edit | ||
| (73 intermediate revisions by 38 users not shown) | |||
| Line 1: | Line 1: | ||
| {{DISPLAYTITLE:''N''-Formylmethionine}} | {{DISPLAYTITLE:''N''-Formylmethionine}} | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = |
| verifiedrevid = 448813033 | ||
| | Name = ''N''-Formylmethionine | | Name = ''N''-Formylmethionine | ||
| | ImageFile = N-Formylmethionine. |
| ImageFile = (S)-N-Formylmethionine V.1.svg | ||
| | ImageSize = 200px | | ImageSize = 200px | ||
| | IUPACName = |
| IUPACName = ''N''-Formylmethionine | ||
| |SystematicName=(''S'')-2-Formylamino-4-methylsulfanylbutanoic acid | |||
| | OtherNames = 2-Formylamino-4-methylsulfanyl-butyric acid |
| OtherNames = 2-Formylamino-4-methylsulfanyl-butyric acid; Formylmethionine; ''N''-Formyl(methyl)homocysteine | ||
| | |
|Section1={{Chembox Identifiers | ||
| | abbreviations=fMet | |||
| | Abbreviations=fMet | |||
| ⚫ | | CASNo = 4289-98-9 |
||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| ⚫ | | CASNo = 4289-98-9 | ||
| | ChEBI = 182822 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = PS9357B4XH | |||
| | EINECS = 224-322-8 | | EINECS = 224-322-8 | ||
| | PubChem = 911 | | PubChem = 911 | ||
| | ChemSpiderID=887 | |||
| ⚫ | | SMILES = |
||
| | StdInChI=1S/C6H11NO3S/c1-11-3-2-5(6(9)10)7-4-8/h4-5H,2-3H2,1H3,(H,7,8)(H,9,10) | |||
| | StdInChIKey = PYUSHNKNPOHWEZ-UHFFFAOYSA-N | |||
| ⚫ | | SMILES = CSCC(NC=O)C(O)=O | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | C=6 | H=11 | N=1 | O=3 | S=1 |
| C=6 | H=11 | N=1 | O=3 | S=1 | ||
| | MolarMass = 177.22 g/mol | | MolarMass = 177.22 g/mol | ||
| | Appearance = | | Appearance = | ||
| Line 22: | Line 30: | ||
| | BoilingPt = | | BoilingPt = | ||
| | Solubility = }} | | Solubility = }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | AutoignitionPt = | |||
| | Autoignition = }} | |||
| }} | |||
| |Section7 = {{Chembox Hazards | |||
| | GHS_ref=<ref>{{cite web |title=N-Formyl-DL-methionine |url=https://pubchem.ncbi.nlm.nih.gov/compound/911#section=Safety-and-Hazards |website=pubchem.ncbi.nlm.nih.gov |language=en}}</ref> | |||
| | GHSPictograms = {{GHS07}} | |||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|319}} | |||
| | PPhrases = {{P-phrases|264+265|280|305+351+338|337+317}} | |||
| }} | |||
| }} | }} | ||
| '''''N''-Formylmethionine''' (fMet |
'''''N''-Formylmethionine''' (fMet,<ref>{{Cite web|last=PubChem|title=N-Formyl-DL-methionine|url=https://pubchem.ncbi.nlm.nih.gov/compound/911|access-date=2020-10-24|website=pubchem.ncbi.nlm.nih.gov|language=en}}</ref> HCO-Met,<ref name="iupacaa"></ref> For-Met<ref name="iupacaa" />) is a derivative of the ] ] in which a ] group has been added to the ] group. It is specifically used for initiation of ] from ]l and ] genes, and may be removed ]. | ||
| fMet plays a crucial part in the protein synthesis of bacteria, ] and ]s. It is not used in ]ic protein synthesis of ]s, where eukaryotic ]s are ]. It is also not used by ]. In the human body, fMet is recognized by the immune system as foreign material and stimulates the body to fight against potential infection. | fMet plays a crucial part in the protein synthesis of bacteria, ] and ]s. It is not used in ]ic protein synthesis of ]s, where eukaryotic ]s are ]. It is also not used by ]. In the human body, fMet is recognized by the immune system as foreign material, or as an alarm signal released by damaged cells, and stimulates the body to fight against potential infection. | ||
| ==Function in protein synthesis== | ==Function in protein synthesis== | ||
| fMet is a starting residue in the synthesis of ] in bacteria and eukaryotic organelles, and, consequently, is located at the N-terminus of the growing ]. fMet is delivered to the ] (30S) - mRNA complex by a specialized ] (tRNA<sup>fMet</sup>) which has a 3'-UAC-5' ] that is capable of binding with the 5'-AUG-3' start ] located on the ]. | |||
| === Translation === | |||
| fMet is coded by the same ] as methionine, AUG. However, AUG is also the ] initiation codon. When the codon is used for initiation, fMet is used instead of methionine, thereby forming the first amino acid of the nascent ] chain. When the same codon appears later in the ], normal methionine is used. Many organisms use variations of this basic mechanism. | |||
| fMet is required for efficient initiation of protein synthesis in most groups of bacteria. The 30S ribosome–mRNA complex specifically recruits tRNAs with a formylated amino acid – tRNA<sup>fMet</sup> attached to fMet in the natural case.<ref name=p28204695>{{cite journal |last1=Shetty |first1=S |last2=Shah |first2=RA |last3=Chembazhi |first3=UV |last4=Sah |first4=S |last5=Varshney |first5=U |title=Two highly conserved features of bacterial initiator tRNAs license them to pass through distinct checkpoints in translation initiation. |journal=Nucleic Acids Research |date=28 February 2017 |volume=45 |issue=4 |pages=2040–2050 |doi=10.1093/nar/gkw854 |pmid=28204695 |pmc=5389676}}</ref> | |||
| Because the fMet directs initiation, ]s in bacteria start (]) with a fMet residue instead of a methionine. Further occurrences of the "AUG" codon will result in a normal methionine, because a normal "elongating" tRNA<sup>Met</sup> is used.<ref name=p28204695/> | |||
| The addition of the formyl group to methionine is catalyzed by the ] ]. This modification is done after methionine has been loaded onto tRNA<sup>fMet</sup> by ]. | |||
| The addition of the formyl group to methionine is catalyzed by the ] ]. This modification is done after methionine has been loaded onto tRNA<sup>fMet</sup> by ]. Methionine itself can be loaded either onto tRNA<sup>fMet</sup> or tRNA<sup>Met</sup>. However, formyltransferase will catalyze the addition of the formyl group to methionine only if methionine has been loaded onto tRNA<sup>fMet</sup>, not onto tRNA<sup>Met</sup>. This is because the formyltransferase recognizes specific features of tRNA<sup>fMet</sup>.<ref name=p28204695/> | |||
| The ] of ] cells, including those of humans, and the ] of ] cells also initiate protein synthesis with fMet. Given that mitochondria and chloroplasts have this initial protein synthesis with fMet in common with bacteria, this has been cited as evidence for the ].<ref>{{Cite book|last=Alberts, Bruce|url=https://www.worldcat.org/oclc/887605755|title=Molecular biology of the cell|date=18 November 2014|isbn=978-0-8153-4432-2|edition=Sixth|location=New York, NY|pages=800|oclc=887605755}}</ref> | |||
| ⚫ | |||
| Unexpectedly, formyltransferase can also act upon eukaryotic initiator tRNA in living yeast cells. Even under normal conditions, the nuclear-encoded formyltransferase is not completely imported into mitochondria; even more is left in the cytosol under stress. These cytosolic formyltransferase produce fMet-tRNA<sub>i</sub>, which can be used by cytosolic ribosomes to produce proteins with a N-terminal fMet. These proteins are targeted for degradation by specific processes in the cell.<ref name=degron>{{cite journal |last1=Varshavsky |first1=Alexander |title=N-degron and C-degron pathways of protein degradation |journal=Proceedings of the National Academy of Sciences |date=8 January 2019 |volume=116 |issue=2 |pages=358–366 |doi=10.1073/pnas.1816596116 |doi-access=free |pmid=30622213 |pmc=6329975|bibcode=2019PNAS..116..358V }}</ref> | |||
| === Further processing === | |||
| ⚫ | The ''N''-terminal fMet is removed from majority of proteins, both host and recombinant, by a sequence of two enzymatic reactions. First, ] (PDF) deformylates it, converting the residue back to a normal methionine. Then ] (MetAP) removes the residue from the chain.<ref name="pmid3024631">{{cite journal |vauthors=Sherman F, Stewart JW, Tsunasawa S |title=Methionine or not methionine at the beginning of a protein |journal=] |volume=3 |issue=1 |pages=27–31 |date=July 1985 |pmid=3024631 |doi=10.1002/bies.950030108 |s2cid=33735710 }}</ref> MetAP only acts on proteins with second-position residues that are less bulky than valine.<ref name=p26866044/> | ||
| The ''N''-terminal fMet, if not removed by PDF, seems to act as a ], a signal for protein degradation.<ref name=p26866044>{{cite journal |last1=Piatkov |first1=KI |last2=Vu |first2=TT |last3=Hwang |first3=CS |last4=Varshavsky |first4=A |title=Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. |journal=Microbial Cell (Graz, Austria) |date=2015 |volume=2 |issue=10 |pages=376–393 |doi=10.15698/mic2015.10.231 |pmid=26866044 |pmc=4745127}}</ref> | |||
| === Variation === | |||
| The formyl group is not strictly required for initiation. Bacteria with their formyltransferase knocked out, which prevents Met-tRNA<sup>fMet</sup> (i.e. methionine loaded onto tRNA<sup>fMet</sup>) from turning into fMet-tRNA<sup>fMet</sup>, can have varying degrees of residual ability to start protein synthesis. ''E. coli'', ''S. pneumoniae'' and ''B. subtilis'' show almost no remaining translation ability, while ''P. aeruginosa'', ''S. aureus'','' H. influenzae'', and possibly ''S. faecalis'' still churn out plenty of protein. In ''P. aeruginosa'', this ability is facilitated by ], which can carry both Met-tRNA<sup>fMet</sup> and fMet-tRNA<sup>fMet</sup> to the ribosome.<ref>{{cite journal |last1=Piatkov |first1=KI |last2=Vu |first2=TT |last3=Hwang |first3=CS |last4=Varshavsky |first4=A |title=Formyl-methionine as a degradation signal at the N-termini of bacterial proteins. |journal=Microbial Cell (Graz, Austria) |date=2015 |volume=2 |issue=10 |pages=376–393 |doi=10.15698/mic2015.10.231 |pmid=26866044 |doi-access=free|pmc=4745127 }}</ref> | |||
| ==Relevance to immunology== | ==Relevance to immunology== | ||
| Because fMet is present in proteins made by |
Because fMet is present in proteins made by bacteria but not in those made by ] (other than in bacterially derived organelles), the ] might use it to help distinguish self from non-self. ] can bind proteins starting with fMet, and use them to initiate the attraction of circulating blood ] and then stimulate microbicidal activities such as ].<ref>{{GeorgiaImmunology|1/phagstep}}</ref><ref name="urlThe Innate Immune System">{{cite web |url=http://student.ccbcmd.edu/courses/bio141/lecguide/unit4/innate/prr.html |title=The Innate Immune System: Pattern-Recognition Receptors, Antigen-Nonspecific Antimicrobial Body Molecules, and Cytokines |url-status=dead |archive-url=https://web.archive.org/web/20100727061353/http://student.ccbcmd.edu/courses/bio141/lecguide/unit4/innate/prr.html |archive-date=2010-07-27 }}</ref><ref name="pmid2958480">{{cite journal |vauthors=Detmers PA, Wright SD, Olsen E, Kimball B, Cohn ZA |title=Aggregation of complement receptors on human neutrophils in the absence of ligand |journal=] |volume=105 |issue=3 |pages=1137–45 |date=September 1987 |pmid=2958480 |pmc=2114803 |doi= 10.1083/jcb.105.3.1137|url=http://www.jcb.org/cgi/pmidlookup?view=long&pmid=2958480}}</ref> | ||
| Since fMet is present in proteins made by mitochondria and chloroplasts, more recent theories do not see it as a molecule that the immune system can use to distinguish self from non-self.<ref>{{cite journal | title = Circulating mitochondrial DAMPs cause inflammatory responses to injury | journal =Nature | date=Mar 4, 2010 | volume = 464 | issue = 7285 | pages = 104–107 | doi=10.1038/nature08780 | pmid=20203610 | pmc=2843437 | vauthors=Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ| bibcode =2010Natur.464..104Z }}</ref> Instead, fMet-containing ]s and proteins appear to be released by the mitochondria of damaged tissues as well as by damaged bacteria, and can thus qualify as an "alarm" signal, as discussed in the ] of immunity. The prototypical fMet-containing oligopeptide is ] (FMLP) which activates leukocytes and other cell types by binding with these cells' ] (FPR1) and ] (FPR2) ] (see also ]). Acting through these receptors, the fMet-containing oligopeptides and proteins are part of the ]; they function to initiate acute ] responses but under other conditions function to inhibit and resolve these responses. fMet-containing oligopeptides and proteins also function in other physiological and pathological responses. | |||
| == See also == | |||
| * ] | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| Line 53: | Line 87: | ||
| {{DEFAULTSORT:Formylmethionine, N-}} | {{DEFAULTSORT:Formylmethionine, N-}} | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 21:01, 6 November 2024
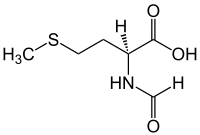
| |
| Names | |
|---|---|
| IUPAC name N-Formylmethionine | |
| Systematic IUPAC name (S)-2-Formylamino-4-methylsulfanylbutanoic acid | |
| Other names 2-Formylamino-4-methylsulfanyl-butyric acid; Formylmethionine; N-Formyl(methyl)homocysteine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | fMet |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H11NO3S |
| Molar mass | 177.22 g/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264+P265, P280, P305+P351+P338, P337+P317 |
| Supplementary data page | |
| N-Formylmethionine (data page) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
N-Formylmethionine (fMet, HCO-Met, For-Met) is a derivative of the amino acid methionine in which a formyl group has been added to the amino group. It is specifically used for initiation of protein synthesis from bacterial and organellar genes, and may be removed post-translationally.
fMet plays a crucial part in the protein synthesis of bacteria, mitochondria and chloroplasts. It is not used in cytosolic protein synthesis of eukaryotes, where eukaryotic nuclear genes are translated. It is also not used by Archaea. In the human body, fMet is recognized by the immune system as foreign material, or as an alarm signal released by damaged cells, and stimulates the body to fight against potential infection.
Function in protein synthesis
Translation
fMet is required for efficient initiation of protein synthesis in most groups of bacteria. The 30S ribosome–mRNA complex specifically recruits tRNAs with a formylated amino acid – tRNA attached to fMet in the natural case.
Because the fMet directs initiation, proteins in bacteria start (N-terminus) with a fMet residue instead of a methionine. Further occurrences of the "AUG" codon will result in a normal methionine, because a normal "elongating" tRNA is used.
The addition of the formyl group to methionine is catalyzed by the enzyme methionyl-tRNA formyltransferase. This modification is done after methionine has been loaded onto tRNA by aminoacyl-tRNA synthetase. Methionine itself can be loaded either onto tRNA or tRNA. However, formyltransferase will catalyze the addition of the formyl group to methionine only if methionine has been loaded onto tRNA, not onto tRNA. This is because the formyltransferase recognizes specific features of tRNA.
The mitochondria of eukaryotic cells, including those of humans, and the chloroplasts of plant cells also initiate protein synthesis with fMet. Given that mitochondria and chloroplasts have this initial protein synthesis with fMet in common with bacteria, this has been cited as evidence for the endosymbiotic theory.
Unexpectedly, formyltransferase can also act upon eukaryotic initiator tRNA in living yeast cells. Even under normal conditions, the nuclear-encoded formyltransferase is not completely imported into mitochondria; even more is left in the cytosol under stress. These cytosolic formyltransferase produce fMet-tRNAi, which can be used by cytosolic ribosomes to produce proteins with a N-terminal fMet. These proteins are targeted for degradation by specific processes in the cell.
Further processing
The N-terminal fMet is removed from majority of proteins, both host and recombinant, by a sequence of two enzymatic reactions. First, peptide deformylase (PDF) deformylates it, converting the residue back to a normal methionine. Then methionine aminopeptidase (MetAP) removes the residue from the chain. MetAP only acts on proteins with second-position residues that are less bulky than valine.
The N-terminal fMet, if not removed by PDF, seems to act as a degron, a signal for protein degradation.
Variation
The formyl group is not strictly required for initiation. Bacteria with their formyltransferase knocked out, which prevents Met-tRNA (i.e. methionine loaded onto tRNA) from turning into fMet-tRNA, can have varying degrees of residual ability to start protein synthesis. E. coli, S. pneumoniae and B. subtilis show almost no remaining translation ability, while P. aeruginosa, S. aureus, H. influenzae, and possibly S. faecalis still churn out plenty of protein. In P. aeruginosa, this ability is facilitated by bacterial initiation factor 2, which can carry both Met-tRNA and fMet-tRNA to the ribosome.
Relevance to immunology
Because fMet is present in proteins made by bacteria but not in those made by eukaryotes (other than in bacterially derived organelles), the immune system might use it to help distinguish self from non-self. Polymorphonuclear cells can bind proteins starting with fMet, and use them to initiate the attraction of circulating blood leukocytes and then stimulate microbicidal activities such as phagocytosis.
Since fMet is present in proteins made by mitochondria and chloroplasts, more recent theories do not see it as a molecule that the immune system can use to distinguish self from non-self. Instead, fMet-containing oligopeptides and proteins appear to be released by the mitochondria of damaged tissues as well as by damaged bacteria, and can thus qualify as an "alarm" signal, as discussed in the Danger model of immunity. The prototypical fMet-containing oligopeptide is N-formylmethionine-leucyl-phenylalanine (FMLP) which activates leukocytes and other cell types by binding with these cells' formyl peptide receptor 1 (FPR1) and formyl peptide receptor 2 (FPR2) G protein coupled receptors (see also formyl peptide receptor 3). Acting through these receptors, the fMet-containing oligopeptides and proteins are part of the innate immune system; they function to initiate acute inflammation responses but under other conditions function to inhibit and resolve these responses. fMet-containing oligopeptides and proteins also function in other physiological and pathological responses.
See also
References
- "N-Formyl-DL-methionine". pubchem.ncbi.nlm.nih.gov.
- PubChem. "N-Formyl-DL-methionine". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-10-24.
- ^ Nomenclature and Symbolism for Amino Acids and Peptides, 3AA-18 and 3AA-19
- ^ Shetty, S; Shah, RA; Chembazhi, UV; Sah, S; Varshney, U (28 February 2017). "Two highly conserved features of bacterial initiator tRNAs license them to pass through distinct checkpoints in translation initiation". Nucleic Acids Research. 45 (4): 2040–2050. doi:10.1093/nar/gkw854. PMC 5389676. PMID 28204695.
- Alberts, Bruce (18 November 2014). Molecular biology of the cell (Sixth ed.). New York, NY. p. 800. ISBN 978-0-8153-4432-2. OCLC 887605755.
{{cite book}}: CS1 maint: location missing publisher (link) - Varshavsky, Alexander (8 January 2019). "N-degron and C-degron pathways of protein degradation". Proceedings of the National Academy of Sciences. 116 (2): 358–366. Bibcode:2019PNAS..116..358V. doi:10.1073/pnas.1816596116. PMC 6329975. PMID 30622213.
- Sherman F, Stewart JW, Tsunasawa S (July 1985). "Methionine or not methionine at the beginning of a protein". BioEssays. 3 (1): 27–31. doi:10.1002/bies.950030108. PMID 3024631. S2CID 33735710.
- ^ Piatkov, KI; Vu, TT; Hwang, CS; Varshavsky, A (2015). "Formyl-methionine as a degradation signal at the N-termini of bacterial proteins". Microbial Cell (Graz, Austria). 2 (10): 376–393. doi:10.15698/mic2015.10.231. PMC 4745127. PMID 26866044.
- Piatkov, KI; Vu, TT; Hwang, CS; Varshavsky, A (2015). "Formyl-methionine as a degradation signal at the N-termini of bacterial proteins". Microbial Cell (Graz, Austria). 2 (10): 376–393. doi:10.15698/mic2015.10.231. PMC 4745127. PMID 26866044.
- Immunology at MCG 1/phagstep
- "The Innate Immune System: Pattern-Recognition Receptors, Antigen-Nonspecific Antimicrobial Body Molecules, and Cytokines". Archived from the original on 2010-07-27.
- Detmers PA, Wright SD, Olsen E, Kimball B, Cohn ZA (September 1987). "Aggregation of complement receptors on human neutrophils in the absence of ligand". The Journal of Cell Biology. 105 (3): 1137–45. doi:10.1083/jcb.105.3.1137. PMC 2114803. PMID 2958480.
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (Mar 4, 2010). "Circulating mitochondrial DAMPs cause inflammatory responses to injury". Nature. 464 (7285): 104–107. Bibcode:2010Natur.464..104Z. doi:10.1038/nature08780. PMC 2843437. PMID 20203610.
External links
- N-Formylmethionine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)