| Revision as of 16:45, 11 June 2011 editLuckas-bot (talk | contribs)929,662 editsm r2.7.1) (robot Adding: bg:Апиоза← Previous edit | Latest revision as of 03:42, 9 August 2020 edit undoGraeme Bartlett (talk | contribs)Administrators249,771 edits more ids | ||
| (19 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 433741882 | ||
| | Name = Apiose | | Name = Apiose | ||
| | ImageFile = D-Apiose structure. |
| ImageFile = D-Apiose structure.svg | ||
| | ImageSize = 150px | | ImageSize = 150px | ||
| | ImageName = Chemical structure of apiose | | ImageName = Chemical structure of apiose | ||
| | IUPACName = 2,3,4- |
| IUPACName = 2,3,4-Trihydroxy-3-(hydroxymethyl)butanal | ||
| | OtherNames = D- |
| OtherNames = <small>D</small>-Apiose<br>3-''C''-(Hydroxymethyl)-<small>D</small>-glycerotetrose<br>Apio-β-<small>D</small>-furanosyl | ||
| |Section1= |
|Section1={{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 16735670 | | ChemSpiderID = 16735670 | ||
| Line 15: | Line 16: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = ASNHGEVAWNWCRQ-LJJLCWGRSA-N | | StdInChIKey = ASNHGEVAWNWCRQ-LJJLCWGRSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 639-97-4 | | CASNo = 639-97-4 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | CASNo_Ref = | |||
| | UNII = E59T26TCEC | |||
| | CASOther = | |||
| | |
| ChEBI = 141215 | ||
| | KEGG = C21040 | |||
| | PubChem = 12306753 | |||
| | SMILES = O1(CO)COC(O)1O | | SMILES = O1(CO)COC(O)1O | ||
| | InChI = 1/C5H10O5/c6-1-5(9)2-10-4(8)3(5)7/h3-4,6-9H,1-2H2/t3-,4?,5+/m0/s1 | | InChI = 1/C5H10O5/c6-1-5(9)2-10-4(8)3(5)7/h3-4,6-9H,1-2H2/t3-,4?,5+/m0/s1 | ||
| | MeSHName = | | MeSHName = | ||
| }} | }} | ||
| |Section2= |
|Section2={{Chembox Properties | ||
| | C=5 | H=10 | O=5 | |||
| | Formula = C<sub>5</sub>H<sub>10</sub>O<sub>5</sub> | |||
| | MolarMass = 150.13 g/mol | |||
| | ExactMass = 150.052823 | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| Line 34: | Line 36: | ||
| }} | }} | ||
| }} | }} | ||
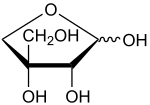
| ''' Apiose''' is a branched-chain ] found as residues in ]s-type ]s; that occurs in ] and many other ]s. | |||
| ''' Apiose''' is a branched-chain ] found as residues in ]s-type ]s; that occurs in ] and many other ]s. Apiose is a component of ] ].<ref>{{Cite journal | doi = 10.1093/glycob/cww012 | pmid = 26848180 | title = Apiose: One of nature's witty games | journal = Glycobiology | volume = 26 | issue = 5 | pages = 430–442 | year = 2016 | last1 = Pičmanová | first1 = Martina | last2 = Møller | first2 = Birger Lindberg | doi-access = free }}</ref> | |||
| ⚫ | ] uses ] and ] to produce |
||
| ⚫ | ] uses ] and ] to produce apiitol-apiose, ], and ]. | ||
| ⚫ | ] uses ] and ] to produce ], ] (apigenin), and |
||
| ⚫ | ] uses ] and ] to produce ], ] (apigenin), and 7-O-β-<small>D</small>-apiosyl-(1->2)-β-apiitol-glucoside. | ||
| ==References== | |||
| {{Reflist}} | |||
| ==External links== | ==External links== | ||
| {{wiktionary}} | {{wiktionary inline}} | ||
| ] | ] | ||
| {{biochemistry-stub}} | |||
| ] | |||
| ] | |||
Latest revision as of 03:42, 9 August 2020
| |
| Names | |
|---|---|
| IUPAC name 2,3,4-Trihydroxy-3-(hydroxymethyl)butanal | |
| Other names
D-Apiose 3-C-(Hydroxymethyl)-D-glycerotetrose Apio-β-D-furanosyl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H10O5 |
| Molar mass | 150.130 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Apiose is a branched-chain sugar found as residues in galacturonans-type pectins; that occurs in parsley and many other plants. Apiose is a component of cell wall polysaccharides.
Apiose 1-reductase uses D-apiitol and NAD to produce apiitol-apiose, NADH, and H.
Flavone apiosyltransferase uses UDP-apiose and 5,7,4'-trihydroxyflavone 7-O-β-D-glucoside to produce UDP, 5,7,4'-trihydroxyflavone (apigenin), and 7-O-β-D-apiosyl-(1->2)-β-apiitol-glucoside.
References
- Pičmanová, Martina; Møller, Birger Lindberg (2016). "Apiose: One of nature's witty games". Glycobiology. 26 (5): 430–442. doi:10.1093/glycob/cww012. PMID 26848180.
External links
![]() The dictionary definition of apiose at Wiktionary
The dictionary definition of apiose at Wiktionary