| Revision as of 18:04, 17 July 2011 editDcirovic (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers253,275 edits →External links← Previous edit |
Latest revision as of 12:25, 5 September 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,127 edits removed Category:Fluoroarenes; added Category:4-Fluorophenyl compounds using HotCat |
| (23 intermediate revisions by 20 users not shown) |
| Line 1: |
Line 1: |
|
|
{{short description|Chemical compound}} |
|
{{chembox |
|
|
|
{{Chembox |
| ⚫ |
| verifiedrevid = 376944058 |
|
|
|
| Verifiedfields = changed |
| ⚫ |
| ImageFile = LY334370.png |
|
|
|
| Watchedfields = changed |
|
| ImageSize = 200px |
|
|
⚫ |
| verifiedrevid = 439982052 |
| ⚫ |
| IUPACName = 4-fluoro-''N''-benzamide |
|
|
⚫ |
| ImageFile = LY334370.svg |
|
|
| ImageFile2 = LY-334370 3D BS.png |
|
⚫ |
| PIN = 4-Fluoro-''N''-benzamide |
|
| OtherNames = LY-334,370 |
|
| OtherNames = LY-334,370 |
|
| Section1 = {{Chembox Identifiers |
|
|Section1={{Chembox Identifiers |
|
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo=182563-08-2 |
|
| CASNo = 182563-08-2 |
| ⚫ |
| PubChem=5311258 |
|
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| IUPHAR_ligand = 151 |
|
|
|
| UNII = 5Q7I1WL2UY |
|
⚫ |
| PubChem = 5311258 |
|
|
| IUPHAR_ligand2 = 151 |
|
| IUPHAR_ligand = 20 |
|
| IUPHAR_ligand = 20 |
|
| SMILES=CN1CCC(c2c3cc(N()C(c4ccc(F)cc4)=O)ccc3n()c2)CC1 |
|
| SMILES = CN1CCC(c2c3cc(N()C(c4ccc(F)cc4)=O)ccc3n()c2)CC1 |
|
|
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
| ⚫ |
}} |
|
|
|
| ChemSpiderID = 4470773 |
| ⚫ |
| Section2 = {{Chembox Properties |
|
|
|
| InChI = 1/C21H22FN3O/c1-25-10-8-14(9-11-25)19-13-23-20-7-6-17(12-18(19)20)24-21(26)15-2-4-16(22)5-3-15/h2-7,12-14,23H,8-11H2,1H3,(H,24,26) |
|
| Formula=C<sub>21</sub>H<sub>22</sub>FN<sub>3</sub>O |
|
|
|
| InChIKey = MDMJLMDBRQXOOI-UHFFFAOYAA |
|
| MolarMass=351.42 g/mol |
|
|
|
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|
| Appearance= |
|
|
|
| StdInChI = 1S/C21H22FN3O/c1-25-10-8-14(9-11-25)19-13-23-20-7-6-17(12-18(19)20)24-21(26)15-2-4-16(22)5-3-15/h2-7,12-14,23H,8-11H2,1H3,(H,24,26) |
|
| Density= |
|
|
|
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|
| MeltingPt= |
|
|
|
| StdInChIKey = MDMJLMDBRQXOOI-UHFFFAOYSA-N |
|
| BoilingPt= |
|
|
|
| RTECS = |
| ⚫ |
| Solubility= |
|
|
|
| MeSHName = C108218 |
| ⚫ |
}} |
|
|
⚫ |
}} |
| ⚫ |
| Section3 = {{Chembox Hazards |
|
|
⚫ |
|Section2={{Chembox Properties |
|
| MainHazards= |
|
|
|
| C=21 | H=22 | F=1 | N=3 | O=1 |
|
| FlashPt= |
|
|
|
| Appearance = |
|
| Autoignition= |
|
|
|
| Density = |
| ⚫ |
}} |
|
|
|
| MeltingPt = |
|
|
| BoilingPt = |
|
⚫ |
| Solubility = |
|
⚫ |
}} |
|
⚫ |
|Section3={{Chembox Hazards |
|
|
| MainHazards = |
|
|
| FlashPt = |
|
|
| AutoignitionPt = |
|
⚫ |
}} |
|
}} |
|
}} |
|
|
|
|
|
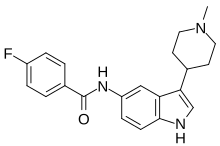
'''LY-334,370''' is a selective ] receptor ]<ref name="pmid9641544">{{cite journal | author = Dupuis DS, Colpaert FC, Pauwels PJ | title = G-protein activation at 5-HT<sub>1A</sub> receptors by the 5-HT<sub>1F</sub> ligand LY-334,370 in guinea-pig brain sections and recombinant cell lines | journal = Br. J. Pharmacol. | volume = 124 | issue = 2 | pages = 283–90 | year = 1998 | pmid = 9641544 | doi = 10.1038/sj.bjp.0701832 | issn = }}</ref> which was under development by ] for the treatment of ] headaches.<ref name="pmid10668103">{{cite journal | author = Shepheard S, Edvinsson L, Cumberbatch M, Williamson D, Mason G, Webb J, Boyce S, Hill R, Hargreaves R | title = Possible antimigraine mechanisms of action of the 5HT<sub>1F</sub> receptor agonist LY-334,370 | journal = Cephalalgia | volume = 19 | issue = 10 | pages = 851–8 | year = 1999 | pmid = 10668103 | doi = 10.1046/j.1468-2982.1999.1910851.x | issn = }}</ref> The drug showed efficacy in a phase II clinical trial<ref name="pmid11675061">{{cite journal | author = Goldstein DJ, Roon KI, Offen WW, Ramadan NM, Phebus LA, Johnson KW, Schaus JM, Ferrari MD | title = Selective seratonin 1F (5-HT<sub>1F</sub>) receptor agonist LY334370 for acute migraine: a randomised controlled trial | journal = Lancet | volume = 358 | issue = 9289 | pages = 1230–4 | year = 2001 | pmid = 11675061 | doi = 10.1016/S0140-6736(01)06347-4 | issn = }}</ref> but further development was halted due to toxicity detected in animals.<ref name="pmid16797716">{{cite journal | author = Ramadan NM, Buchanan TM | title = New and future migraine therapy | journal = Pharmacol. Ther. | volume = 112 | issue = 1 | pages = 199–212 | year = 2006 | pmid = 16797716 | doi = 10.1016/j.pharmthera.2005.04.010 | issn = }}</ref> |
|
'''LY-334370''' is a selective ] ]<ref name="pmid9641544">{{cite journal |vauthors=Dupuis DS, Colpaert FC, Pauwels PJ | title = G-protein activation at 5-HT<sub>1A</sub> receptors by the 5-HT<sub>1F</sub> ligand LY-334,370 in guinea-pig brain sections and recombinant cell lines | journal = Br. J. Pharmacol. | volume = 124 | issue = 2 | pages = 283–90 | year = 1998 | pmid = 9641544 | doi = 10.1038/sj.bjp.0701832 | pmc=1565387}}</ref> which was under development by ] for the treatment of ] headaches.<ref name="pmid10668103">{{cite journal |vauthors=Shepheard S, Edvinsson L, Cumberbatch M, Williamson D, Mason G, Webb J, Boyce S, Hill R, Hargreaves R | title = Possible antimigraine mechanisms of action of the 5HT<sub>1F</sub> receptor agonist LY-334,370 | journal = Cephalalgia | volume = 19 | issue = 10 | pages = 851–8 | year = 1999 | pmid = 10668103 | doi = 10.1046/j.1468-2982.1999.1910851.x | s2cid = 8439008 }}</ref> The drug showed efficacy in a phase II clinical trial<ref name="pmid11675061">{{cite journal |vauthors=Goldstein DJ, Roon KI, Offen WW, Ramadan NM, Phebus LA, Johnson KW, Schaus JM, Ferrari MD | title = Selective seratonin 1F (5-HT<sub>1F</sub>) receptor agonist LY334370 for acute migraine: a randomised controlled trial | journal = Lancet | volume = 358 | issue = 9289 | pages = 1230–4 | year = 2001 | pmid = 11675061 | doi = 10.1016/S0140-6736(01)06347-4 | s2cid = 39926402 }}</ref> but further development was halted due to toxicity detected in animals.<ref name="pmid16797716">{{cite journal |vauthors=Ramadan NM, Buchanan TM | title = New and future migraine therapy | journal = Pharmacol. Ther. | volume = 112 | issue = 1 | pages = 199–212 | year = 2006 | pmid = 16797716 | doi = 10.1016/j.pharmthera.2005.04.010 }}</ref> |
|
|
|
|
|
==See also== |
|
==See also== |
|
|
* ] |
|
* ], another 5-HT<sub>1F</sub> agonist for the treatment of migraine |
|
|
|
* ] |
|
|
* ] |
|
|
|
|
|
==References== |
|
==References== |
| Line 39: |
Line 56: |
|
* {{MeshName|4-fluoro-N-(3-(1-methyl-4-piperidinyl)-1H-indol-5-yl)benzamide}} |
|
* {{MeshName|4-fluoro-N-(3-(1-methyl-4-piperidinyl)-1H-indol-5-yl)benzamide}} |
|
|
|
|
|
|
{{Serotonin receptor modulators}} |
|
{{Serotonergics}} |
|
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
] |
|
|
|
|
|
{{nervous-system-drug-stub}} |
|
{{nervous-system-drug-stub}} |
|
|
|
|
] |
|