| Revision as of 06:53, 28 July 2011 editWoohookitty (talk | contribs)Administrators611,228 editsm WPCleaner (v1.09) Repaired link to disambiguation page - (You can help) - Native Americans← Previous edit | Latest revision as of 06:15, 12 July 2024 edit undoحسن علي البط (talk | contribs)Extended confirmed users, Pending changes reviewers19,940 edits added Category:Secondary alcohols using HotCat | ||
| (87 intermediate revisions by 44 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 441829253 | ||
| | Name = Betulin | | Name = Betulin | ||
| | ImageFile = Betulin. |
| ImageFile = Betulin.svg | ||
| | |
| ImageName = Betulin | ||
| | IUPACName = Lup-20(29)-ene-3β,28-diol | | IUPACName = Lup-20(29)-ene-3β,28-diol | ||
| | SystematicName = (1''R'',3a''S'',5a''R'',5b''R'',7a''R'',9''S'',11a''R'',11b''R'',13a''R'',13b''R'')-3a-(Hydroxymethyl)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-1''H''-cyclopentachrysen-9-ol | |||
| | OtherNames = | |||
| | OtherNames = Betulinol, betuline, betulol, betulinic alcohol, trochol | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | |
| InChI = 1/C30H50O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h20-25,31-32H,1,8-18H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1 | ||
| | |
| PubChem = 72326 | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 23236 | | ChEMBL = 23236 | ||
| Line 21: | Line 21: | ||
| | StdInChIKey = FVWJYYTZTCVBKE-ROUWMTJPSA-N | | StdInChIKey = FVWJYYTZTCVBKE-ROUWMTJPSA-N | ||
| | CASNo = 473-98-3 | | CASNo = 473-98-3 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = 6W70HN7X7O | |||
| ⚫ | | |
||
| ⚫ | | EC_number = 207-475-5 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 65272 | | ChemSpiderID = 65272 | ||
| | KEGG_Ref = {{keggcite| |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C08618 | | KEGG = C08618 | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Appearance = solid with needle-like crystals<ref name=":crc">{{Cite book|chapter-url=https://books.google.com/books?id=bNDMBQAAQBAJ&pg=SA7-PA|title=CRC Handbook of Chemistry and Physics|last1=Haynes|first1=William M.|last2=Lide|first2=David R.|last3=Bruno|first3=Thomas J.|publisher=CRC Press|year=2014|isbn=9781482208689|edition=95th|location=Boca Raton, Florida|pages=340|chapter=3|oclc=908078665}}</ref> | |||
| | |
| C=30 | H=50 | O=2 | ||
| | MeltingPt = 256–257 °C | |||
| | MeltingPtC = 256 to 257 | |||
| | Solubility = insoluble<ref name=":crc" /> | |||
| | SolubleOther = slightly soluble in ] and ]; soluble in ], ] and ]<ref name=":crc" /> | |||
| }} | }} | ||
| | Section3 = | |||
| | Section4 = | |||
| | Section5 = | |||
| | Section6 = | |||
| }} | }} | ||
| '''Betulin''' (lup-20(29)-ene-3β,28-diol) is an abundant naturally occurring ]. It is commonly isolated from the ] of ] trees and forms up to 30% of the dry weight of the extractive <ref>{{citation | title = Isolation of Betulin and Rearrangement to Allobetulin A Biomimetic Natural Product Synthesis | first1 = Brian | last1 = Green | first2 = Michael D. | last2 = Bentley | first3 = Bong Y. | last3 = Chung | first4 = Nicholas G. | last4 = Lynch | first5 = Bruce L. | last5 = Jensen | journal = J. Chem. Educ. | year = 1985 | volume = 200 | pages = 7}}.</ref>. The purpose of the compound in the bark is not known. It can be converted to ] (the ] group replaced by a ] group), which is biologically more active than betulin itself. | |||
| '''Betulin''' is an abundant, naturally occurring ]. It is commonly isolated from the ] of ] trees. It forms up to 30% of the dry weight of ] bark.<ref>{{Cite journal|last1=Green|first1=Brian|last2=Bentley|first2=Michael D.|last3=Chung|first3=Bong Y.|last4=Lynch|first4=Nicholas G.|last5=Jensen|first5=Bruce L.|date=2007-12-01|title=Isolation of Betulin and Rearrangement to Allobetulin. A Biomimetic Natural Product Synthesis|journal=Journal of Chemical Education|language=EN|volume=84|issue=12|pages=1985|doi=10.1021/ed084p1985|bibcode=2007JChEd..84.1985G}}</ref> It is also found in ].{{Citation needed|date=April 2018}} '']'' contains betulin.<ref>{{Cite journal|last1=Gao|first1=Yuan|last2=Xu|first2=Hongyu|last3=Lu|first3=Zhenming|last4=Xu|first4=Zhenghong|date=November 2009|title=Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of ''Inonotus obliquus''|journal=Se Pu |volume=27|issue=6|pages=745–749|issn=1000-8713|pmid=20352924}}</ref> | |||
| The compound in the bark gives the tree its white color which appears to protect the tree from ] overheating by the ]. As a result, birches are some of the northernmost occurring deciduous trees. | |||
| ==History== | |||
| Betulin was discovered in ] by ]-] chemist ].<ref>{{Cite journal|last=Lowitz|first=J. T.|date=1788|title=Űber eine neue, fast benzoeartige substanz der briken|journal=Crell's Chem. Ann.|volume=1|pages=312–317}}</ref><ref>{{Cite journal|last1=Król|first1=Sylwia Katarzyna|last2=Kiełbus|first2=Michał|last3=Rivero-Müller|first3=Adolfo|last4=Stepulak|first4=Andrzej|date=2015|title=Comprehensive Review on Betulin as a Potent Anticancer Agent |journal=BioMed Research International|volume=2015|page=584189 |doi=10.1155/2015/584189 |pmc=4383233|pmid=25866796|doi-access=free }}</ref> | |||
| ==Chemistry== | ==Chemistry== | ||
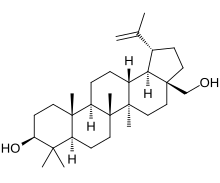
| Chemically, betulin is a triterpenoid of ] structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28. | Chemically, betulin is a triterpenoid of ] structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28. | ||
| == See also == | |||
| ==Biological activities== | |||
| '']'' contains betulin,<ref name="pmid20352924">{{cite journal |last1=Gao |first1=Y |last2=Xu |first2=H |last3=Lu |first3=Z |last4=Xu |first4=Z |title=Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of Inonotus obliquus |journal=Se pu |volume=27 |issue=6 |pages=745–9 |year=2009 |pmid=20352924}}</ref> | |||
| * ] | |||
| Recent clinical studies{{By whom|date=August 2010}} have verified that ] ''(])'' contains betulin and ], compounds shown to be effective against a variety of ]s. ] used red alder bark to treat ], ]s, and ]. ] used an ] made from the bark of red alder to treat ] and ].<ref>{{citation | first = Gregory L. | last = Tilford | title = Edible and Medicinal Plants of the West | publisher = Mountain Press | location = Missoula, MO | year = 1997 | isbn = 0-87842-359-1}}.</ref> | |||
| * ] | |||
| * ]s | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| ==Literature== | |||
| * Franziska B. Mullauer, Jan H. Kessler, Jan Paul Medema , 2009 | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 06:15, 12 July 2024
| |
| Names | |
|---|---|
| IUPAC name Lup-20(29)-ene-3β,28-diol | |
| Systematic IUPAC name (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a-(Hydroxymethyl)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopentachrysen-9-ol | |
| Other names Betulinol, betuline, betulol, betulinic alcohol, trochol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.797 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C30H50O2 |
| Molar mass | 442.728 g·mol |
| Appearance | solid with needle-like crystals |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
| Solubility in water | insoluble |
| Solubility | slightly soluble in ethanol and benzene; soluble in diethyl ether, ethyl acetate and ligroin |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Betulin is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees. It forms up to 30% of the dry weight of silver birch bark. It is also found in birch sap. Inonotus obliquus contains betulin.
The compound in the bark gives the tree its white color which appears to protect the tree from mid-winter overheating by the sun. As a result, birches are some of the northernmost occurring deciduous trees.
History
Betulin was discovered in 1788 by German-Russian chemist Johann Tobias Lowitz.
Chemistry
Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28.
See also
References
- ^ Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2014). "3". CRC Handbook of Chemistry and Physics (95th ed.). Boca Raton, Florida: CRC Press. p. 340. ISBN 9781482208689. OCLC 908078665.
- Green, Brian; Bentley, Michael D.; Chung, Bong Y.; Lynch, Nicholas G.; Jensen, Bruce L. (2007-12-01). "Isolation of Betulin and Rearrangement to Allobetulin. A Biomimetic Natural Product Synthesis". Journal of Chemical Education. 84 (12): 1985. Bibcode:2007JChEd..84.1985G. doi:10.1021/ed084p1985.
- Gao, Yuan; Xu, Hongyu; Lu, Zhenming; Xu, Zhenghong (November 2009). "Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of Inonotus obliquus". Se Pu. 27 (6): 745–749. ISSN 1000-8713. PMID 20352924.
- Lowitz, J. T. (1788). "Űber eine neue, fast benzoeartige substanz der briken". Crell's Chem. Ann. 1: 312–317.
- Król, Sylwia Katarzyna; Kiełbus, Michał; Rivero-Müller, Adolfo; Stepulak, Andrzej (2015). "Comprehensive Review on Betulin as a Potent Anticancer Agent". BioMed Research International. 2015: 584189. doi:10.1155/2015/584189. PMC 4383233. PMID 25866796.