| Revision as of 07:29, 29 July 2011 editSome standardized rigour (talk | contribs)1,905 editsm added alternative spelling← Previous edit | Latest revision as of 21:14, 16 November 2023 edit undoMembraneHacker (talk | contribs)1 editm →HistoryTag: Visual edit | ||
| (44 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Context|date=November 2009}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 442000463 | ||
| | ImageFile = Heme c.svg | | ImageFile = Heme c.svg | ||
| | ImageSize = 250px | | ImageSize = 250px | ||
| | IUPACName = | | IUPACName = | ||
| | OtherNames = | | OtherNames = | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 26598-29-8 | |||
| | |
| CASNo = 26598-29-8 | ||
| | |
| PubChem = 11987638 | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ⚫ | | |
||
| | ChemSpiderID = 10160119 | |||
| | SMILES = OC(=O)CC/c6c(\C)c3n7c6cc2c(/CCC(O)=O)c(/C)c1cc5n8c(cc4n(78n12)c(c=3)c(C(S)=C)c4c)c(\C(S)=C)c5\C | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C34H34N4O4S2.Fe/c1-15-21(7-9-31(39)40)27-14-28-22(8-10-32(41)42)16(2)24(36-28)12-29-34(20(6)44)18(4)26(38-29)13-30-33(19(5)43)17(3)25(37-30)11-23(15)35-27;/h11-14H,5-10H2,1-4H3,(H6,35,36,37,38,39,40,41,42,43,44);/q;+2/p-2/b23-11-,24-12-,25-11-,26-13-,27-14-,28-14-,29-12-,30-13-; | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = KWLVFEFHZOXGTI-IDTMDVKXSA-L | |||
| ⚫ | | MeSHName = heme+C | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>34</sub>H<sub>36</sub>O<sub>4</sub>N<sub>4</sub>S<sub>2</sub>Fe | ||
| | |
| MolarMass = 684.64904 g/mol | ||
| | |
| Appearance = | ||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | Autoignition = | |||
| }} | }} | ||
| }} | }} | ||
| '''Heme C''' (or '''haem C''') is an important kind of ]. | |||
| ⚫ | |||
| ==History== | |||
| The thioether linkages are commonly arranged with two amino acids between the two cysteinyl groups bound to the heme. This is often written as CXXC, within the amino acid sequence presentation with X being most any common amino acid. Vertebrate c type cytochromes also arrange a ] amino acid next to this sequence so that CXXCH represents the five key amino acids associated with the thioether linkages in these proteins. The two thioether bonds are made to heme ring positions 2 and 4, but a few c type cytochromes have hemes with only a single thioether bond.<ref>{{cite journal | doi=10.1098/rstb.2002.1192 | author=Allen, J.W.A., Daltrop, O., Stevens, J.M., Ferguson, S.J.| title=C-type cytochromes: diverse structures and biogenisis systems pose evolutionary problems| journal=Phil. Trans. R. Soc. Lond. B| year=2003 | volume=358 | pages= 255–266 }}</ref> | |||
| ⚫ | The correct structure of heme C was published in mid 20th century by the Swedish biochemist K.-G. Paul.<ref>{{cite journal | doi=10.3891/acta.chem.scand.04-0239 | author=Paul, K.G. | last2=Högfeldt | first2=Erik | last3=Sillén | first3=Lars Gunnar | last4=Kinell | first4=Per-Olof| title=The splitting with silver salts of the cysteine-porphyrin bonds in cytochrome c | journal=Acta Chemica Scandinavica| year=1950 | volume=4 | pages= 239–244| doi-access=free }}</ref> This work confirmed the structure first inferred by the great Swedish biochemist ]. The structure of heme C, based upon NMR and IR experiments of the reduced Fe(II) form of the heme, was confirmed in 1975.<ref>{{cite journal | author1=Caughey, W.S.| author2=Smythe, G.A. | author3=O'Keeffe, D.H. | author4=Maskasky, J.E. | author5=Smith, M.L. | year=1975 | title=Heme A of Cytochrome c Oxidase | journal=] | volume=250 | issue=19 | pages=7602–7622 | doi=10.1016/S0021-9258(19)40860-0 | pmid= 170266| doi-access=free }}</ref> The structure of heme C including the absolute ] configuration about the thioether bonds was first presented for the vertebrate protein, cytochrome c<ref>{{cite journal |author1=Takano T. |author2=Trus B.L. |author3=Mandel N. |author4=Mandel G. |author5=Kallai O.B. |author6=Swanson R. |author7=Dickerson R.E. | year=1977 | title=Tuna cytochrome c at 2.0 A resolution. II. Ferrocytochrome structure analysis. | journal=] | volume=252 |issue=2 | pages=776–785 |doi=10.1016/S0021-9258(17)32784-9 | pmid=188826|doi-access=free }}</ref> and is now extended to many other heme C containing proteins. | ||
| ==Properties== | |||
| ⚫ | The number of heme C units bound to a holoprotein is highly variable. For vertebrate cells one heme C per protein is the rule but for bacteria this number is often 2, 4, 5, 6 or even 16 heme C groups per holoprotein. It is generally agreed the number and arrangement of heme C groups are related and even required for proper holoprotein function. For instance, those proteins containing several heme C groups are involved with multiple electron transfer reactions, particularly important is the 6 electron reduction required to reduce atmospheric nitrogen into two organic ammonia molecules. It is common for the heme C to amino acid ratio to be high for bacterial ]s, so the interiors of some cytochrome c proteins appear packed with many heme C groups compared with other hemeproteins. Some hemeproteins, often from ]s, may contain five hemes C.<ref name="Gwyer"> |
||
| ⚫ | Heme C differs from ] in that the two ] side chains of heme B are replaced by covalent, ] linkages to the ]. The two ] linkages are typically made by cysteine residues of the protein. These linkages do not allow the heme C to easily dissociate from the ], ], compared with the more easily dissociated heme B that may dissociate from the holoprotein, the heme-protein complex, even under mild conditions. This allows a very wide range of cytochrome c structure and function, with myriad c type ] acting primarily as electron carriers. The redox potential for cytochrome c can also be "fine-tuned" by small changes in protein structure and solvent interaction.<ref>{{cite journal | author1=Berghuis, A.M. | author2= Brayer, G.D.| title=Oxidation state-dependent conformational changes in cytochrome c. | journal=J. Mol. Biol.| year=1992 | volume=223 |issue=4 |pages=959–976 | pmid=1311391 | doi=10.1016/0022-2836(92)90255-i}}</ref> | ||
| ⚫ | The number of heme C units bound to a ] is highly variable. For vertebrate cells one heme C per protein is the rule but for bacteria this number is often 2, 4, 5, 6 or even 16 heme C groups per holoprotein. It is generally agreed the number and arrangement of heme C groups are related and even required for proper holoprotein function. For instance, those proteins containing several heme C groups are involved with multiple electron transfer reactions, particularly important is the 6 electron reduction required to reduce atmospheric nitrogen into two organic ammonia molecules. It is common for the heme C to amino acid ratio to be high for bacterial ]s, so the interiors of some cytochrome c proteins appear packed with many heme C groups compared with other hemeproteins. Some hemeproteins, often from ]s, may contain five hemes C.<ref name="Gwyer">{{cite journal|doi=10.1021/ja054160s | pmid=16248601 | volume=127 | issue=43 | title=Diode or Tunnel-Diode Characteristics? Resolving the Catalytic Consequences of Proton Coupled Electron Transfer in a Multi-Centered Oxidoreductase | year=2005 | journal=Journal of the American Chemical Society | pages=14964–14965 | author=Gwyer James D., Richardson David J., Butt Julea N.}}</ref> The ] is another important enzyme that contains a C type heme. | ||
| ⚫ | The thioether linkages seem to allow a great freedom of function for the holoproteins. In general, the c type cytochromes can be "fine tuned" over a wider range of oxidation-reduction potential than cytochromes b. This may be an important reason why cytochrome c is nearly ubiquitous throughout life. Heme C also plays an important role in ] where just a few molecules of cytoplasmic cytochrome c, which must still contain heme C, leads to programmed cell death.<ref>{{cite journal | doi=10.1039/b717196j | author=Bowman, S.E.J., Bren, K.L.| title=The chemistry and biochemistry of heme C: functional bases for covalent attachment| journal=Nat. Prod. Rep.| year=2008 | volume=25 | issue=6 | pages= 1118–1130 | pmid=19030605 | pmc=2654777 }}</ref> | ||
| ⚫ | The thioether linkages seem to allow a great freedom of function for the holoproteins. In general, the c type cytochromes can be "fine tuned" over a wider range of oxidation-reduction potential than cytochromes b. This may be an important reason why cytochrome c is nearly ubiquitous throughout life. Heme C also plays an important role in ] where just a few molecules of cytoplasmic cytochrome c, which must still contain heme C, leads to programmed cell death.<ref>{{cite journal | doi=10.1039/b717196j | author=Bowman, S.E.J., Bren, K.L.| title=The chemistry and biochemistry of heme C: functional bases for covalent attachment| journal=Nat. Prod. Rep.| year=2008 | volume=25 | issue=6 | pages= 1118–1130 | pmid=19030605 | pmc=2654777 }}</ref> Cytochrome c can be measured in human serum and can be used as a marker for inflammation.<ref>{{cite journal | author1=Eleftheriadis, T. | author2= Pissas, G.| author3=Liakopoulos, V. | author4=Stafanidis, I. | title=Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage. | journal=Front. Immunol.| year=2016 | volume=7 | pages= 279 | pmid=27489552 | doi=10.3389/fimmu.2016.00279 | pmc=4951490| doi-access= free}}</ref> | ||
| ⚫ | In addition to these covalent bonds, the heme iron is also usually coordinated to |
||
| ⚫ | In addition to these equatorial covalent bonds, the heme iron is also usually axially coordinated to the side chains of two ]s, making the iron hexacoordinate. For example, mammalian and tuna ] contain a single heme C that is axially coordinated to side chains of both ] and ].<ref>{{cite journal | author=Yeh, S.R., Han, S., and Rousseau, D.L.| title=Cytochrome c folding and unfolding| journal=Accounts of Chemical Research| year=1998 | volume=31 | issue=11 | pages= 727–735| doi=10.1021/ar970084p}}</ref> Perhaps because of the two covalent bonds holding the heme to the protein, the iron of heme C is sometimes axially ligated to the amino group of ] or even water. | ||
| ⚫ | The correct structure of heme C was published |
||
| ⚫ | ==References== | ||
| ⚫ | <references /> | ||
| ==See also== | ==See also== | ||
| Line 48: | Line 54: | ||
| *] | *] | ||
| *] | *] | ||
| ⚫ | ==References== | ||
| ⚫ | <references /> | ||
| {{Enzyme cofactors}} | {{Enzyme cofactors}} | ||
Latest revision as of 21:14, 16 November 2023
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| MeSH | heme+C |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C34H36O4N4S2Fe |
| Molar mass | 684.64904 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
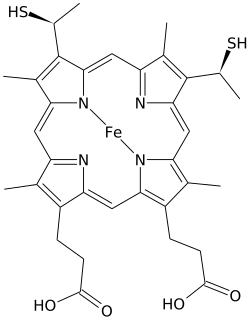
Heme C (or haem C) is an important kind of heme.
History
The correct structure of heme C was published in mid 20th century by the Swedish biochemist K.-G. Paul. This work confirmed the structure first inferred by the great Swedish biochemist Hugo Theorell. The structure of heme C, based upon NMR and IR experiments of the reduced Fe(II) form of the heme, was confirmed in 1975. The structure of heme C including the absolute stereochemical configuration about the thioether bonds was first presented for the vertebrate protein, cytochrome c and is now extended to many other heme C containing proteins.
Properties
Heme C differs from heme B in that the two vinyl side chains of heme B are replaced by covalent, thioether linkages to the apoprotein. The two thioether linkages are typically made by cysteine residues of the protein. These linkages do not allow the heme C to easily dissociate from the holoprotein, cytochrome c, compared with the more easily dissociated heme B that may dissociate from the holoprotein, the heme-protein complex, even under mild conditions. This allows a very wide range of cytochrome c structure and function, with myriad c type cytochromes acting primarily as electron carriers. The redox potential for cytochrome c can also be "fine-tuned" by small changes in protein structure and solvent interaction.
The number of heme C units bound to a holoprotein is highly variable. For vertebrate cells one heme C per protein is the rule but for bacteria this number is often 2, 4, 5, 6 or even 16 heme C groups per holoprotein. It is generally agreed the number and arrangement of heme C groups are related and even required for proper holoprotein function. For instance, those proteins containing several heme C groups are involved with multiple electron transfer reactions, particularly important is the 6 electron reduction required to reduce atmospheric nitrogen into two organic ammonia molecules. It is common for the heme C to amino acid ratio to be high for bacterial hemeproteins, so the interiors of some cytochrome c proteins appear packed with many heme C groups compared with other hemeproteins. Some hemeproteins, often from single cell organisms, may contain five hemes C. The bc1 complex is another important enzyme that contains a C type heme.
The thioether linkages seem to allow a great freedom of function for the holoproteins. In general, the c type cytochromes can be "fine tuned" over a wider range of oxidation-reduction potential than cytochromes b. This may be an important reason why cytochrome c is nearly ubiquitous throughout life. Heme C also plays an important role in apoptosis where just a few molecules of cytoplasmic cytochrome c, which must still contain heme C, leads to programmed cell death. Cytochrome c can be measured in human serum and can be used as a marker for inflammation.
In addition to these equatorial covalent bonds, the heme iron is also usually axially coordinated to the side chains of two amino acids, making the iron hexacoordinate. For example, mammalian and tuna cytochrome c contain a single heme C that is axially coordinated to side chains of both histidine and methionine. Perhaps because of the two covalent bonds holding the heme to the protein, the iron of heme C is sometimes axially ligated to the amino group of lysine or even water.
See also
References
- Paul, K.G.; Högfeldt, Erik; Sillén, Lars Gunnar; Kinell, Per-Olof (1950). "The splitting with silver salts of the cysteine-porphyrin bonds in cytochrome c". Acta Chemica Scandinavica. 4: 239–244. doi:10.3891/acta.chem.scand.04-0239.
- Caughey, W.S.; Smythe, G.A.; O'Keeffe, D.H.; Maskasky, J.E.; Smith, M.L. (1975). "Heme A of Cytochrome c Oxidase". Journal of Biological Chemistry. 250 (19): 7602–7622. doi:10.1016/S0021-9258(19)40860-0. PMID 170266.
- Takano T.; Trus B.L.; Mandel N.; Mandel G.; Kallai O.B.; Swanson R.; Dickerson R.E. (1977). "Tuna cytochrome c at 2.0 A resolution. II. Ferrocytochrome structure analysis". Journal of Biological Chemistry. 252 (2): 776–785. doi:10.1016/S0021-9258(17)32784-9. PMID 188826.
- Berghuis, A.M.; Brayer, G.D. (1992). "Oxidation state-dependent conformational changes in cytochrome c.". J. Mol. Biol. 223 (4): 959–976. doi:10.1016/0022-2836(92)90255-i. PMID 1311391.
- Gwyer James D., Richardson David J., Butt Julea N. (2005). "Diode or Tunnel-Diode Characteristics? Resolving the Catalytic Consequences of Proton Coupled Electron Transfer in a Multi-Centered Oxidoreductase". Journal of the American Chemical Society. 127 (43): 14964–14965. doi:10.1021/ja054160s. PMID 16248601.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bowman, S.E.J., Bren, K.L. (2008). "The chemistry and biochemistry of heme C: functional bases for covalent attachment". Nat. Prod. Rep. 25 (6): 1118–1130. doi:10.1039/b717196j. PMC 2654777. PMID 19030605.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stafanidis, I. (2016). "Cytochrome c as a Potentially Clinical Useful Marker of Mitochondrial and Cellular Damage". Front. Immunol. 7: 279. doi:10.3389/fimmu.2016.00279. PMC 4951490. PMID 27489552.
- Yeh, S.R., Han, S., and Rousseau, D.L. (1998). "Cytochrome c folding and unfolding". Accounts of Chemical Research. 31 (11): 727–735. doi:10.1021/ar970084p.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
| Enzyme cofactors | |||||||
|---|---|---|---|---|---|---|---|
| Active forms |
| ||||||
| Base forms | |||||||