| Revision as of 21:46, 6 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi← Previous edit | Latest revision as of 13:32, 26 September 2024 edit undoMarbletan (talk | contribs)Extended confirmed users5,416 edits unnecessary and unwieldy - a curated handful of examples is sufficient | ||
| (76 intermediate revisions by 46 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| |ImageFile=Azepan.png | |||
| ⚫ | | verifiedrevid = 444338541 | ||
| |ImageSize=100 | |||
| | ImageFileL1 =Azepane.svg | |||
| |IUPACName=azepane | |||
| | ImageSizeL1 =100 | |||
| |OtherNames=Hexahydroazepine | |||
| | ImageAltL1 = Skeletal formula of azepane | |||
| | ImageFileR1 = Azepane-3D-balls.png | |||
| | ImageSizeR1 = 140 | |||
| | ImageAltR1 = Ball-and-stick model of the azepane molecule | |||
| | PIN =Azepane | |||
| | OtherNames ={{bulletedlist|Hexahydroazepine | Hexamethyleneimine | Homopiperidine | Perhydroazepine|HMI| | |||
| azacycloheptane}} | |||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 7828 | | ChemSpiderID = 7828 | ||
| | InChI = 1/C6H13N/c1-2-4-6-7-5-3-1/h7H,1-6H2 | | InChI = 1/C6H13N/c1-2-4-6-7-5-3-1/h7H,1-6H2 | ||
| Line 15: | Line 22: | ||
| | StdInChIKey = ZSIQJIWKELUFRJ-UHFFFAOYSA-N | | StdInChIKey = ZSIQJIWKELUFRJ-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo=111-49-9 | | CASNo =111-49-9 | ||
| ⚫ | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | PubChem=8119 | |||
| | UNII = CZD076G73R | |||
| ⚫ | | |
||
| | PubChem =8119 | |||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | ChEMBL = 1375444 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 32616 | | ChEBI = 32616 | ||
| | SMILES = C1CCCNCC1 | | SMILES = C1CCCNCC1 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
| C=6 | H=13 | N=1 | ||
| | |
| Appearance = colorless liquid | ||
| | Density =0.88 g/cm<sup>3</sup><ref name=sigmaaldrich>{{cite web | url = http://www.sigmaaldrich.com/catalog/product/aldrich/h10401 | title = Hexamethyleneimine}}</ref> | |||
| | Density=0.825 g/cm<sup>3</sup> | |||
| | MeltingPtC = -37 | |||
| | MeltingPt= | |||
| | BoilingPtC = 138 | |||
| | BoilingPt=142.666 °C | |||
| | BoilingPt_notes = (749 mmHg) | |||
| | Solubility= | |||
| | BoilingPt_ref= <ref name=sigmaaldrich/> | |||
| | Solubility = | |||
| }} | }} | ||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | FlashPtC = 30 | |||
| | FlashPt=18.333 °C | |||
| | AutoignitionPtC = | |||
| | Autoignition= | |||
| }} | |||
| }} | }} | ||
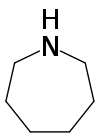
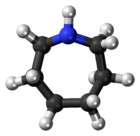
| '''Azepane''' is the ] with the formula (CH<sub>2</sub>)<sub>6</sub>NH. It is a colorless liquid. A cyclic ], it is a precursor to several drugs and pesticides. It is produced by partial ] of ].<ref name=Ullmann>{{cite encyclopedia |author=Karsten Eller |author2=Erhard Henkes |author3=Roland Rossbacher |author4=Hartmut Höke|title=Amines, Aliphatic|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|location=Weinheim|doi=10.1002/14356007.a02_001|isbn=3527306730 }}</ref> | |||
| '''Azepane''' is a saturated ], containing one ] atom in seven-membered ring. | |||
| Like many amines, it reacts with ].<ref>{{cite journal|last1=Sanz-Pérez|first1=E. S.|last2=Arencibia|first2=A.|last3=Sanz|first3=R.|last4=Calleja|first4=G.|title=New developments on carbon dioxide capture using amine-impregnated silicas|journal=Adsorption|issue=4|volume=22|year=2016|pages=366–375|doi=10.1007/s10450-015-9740-2|s2cid=100692983 }}</ref> | |||
| A well known azepane derivative is ]. | |||
| ==Azepane-containing drugs== | |||
| A variety of pharmaceutical drugs contain an azepane ring including ], ], ], ], ], ], and ], among others. | |||
| == See also == | == See also == | ||
| * ] | * ] | ||
| ==References== | |||
| {{reflist}} | |||
| {{heterocyclic-stub}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 13:32, 26 September 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Azepane | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.524 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H13N | ||
| Molar mass | 99.177 g·mol | ||
| Appearance | colorless liquid | ||
| Density | 0.88 g/cm | ||
| Melting point | −37 °C (−35 °F; 236 K) | ||
| Boiling point | 138 °C (280 °F; 411 K) (749 mmHg) | ||
| Hazards | |||
| Flash point | 30 °C (86 °F; 303 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Azepane is the organic compound with the formula (CH2)6NH. It is a colorless liquid. A cyclic secondary amine, it is a precursor to several drugs and pesticides. It is produced by partial hydrogenolysis of hexamethylene diamine.
Like many amines, it reacts with carbon dioxide.
Azepane-containing drugs
A variety of pharmaceutical drugs contain an azepane ring including bazedoxifene, cetiedil, glisoxepide, mecillinam, nabazenil, setastine, and tolazamide, among others.
See also
References
- ^ "Hexamethyleneimine".
- Karsten Eller; Erhard Henkes; Roland Rossbacher; Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- Sanz-Pérez, E. S.; Arencibia, A.; Sanz, R.; Calleja, G. (2016). "New developments on carbon dioxide capture using amine-impregnated silicas". Adsorption. 22 (4): 366–375. doi:10.1007/s10450-015-9740-2. S2CID 100692983.