| Revision as of 16:10, 7 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').← Previous edit |
Latest revision as of 00:32, 24 January 2023 edit undoEntranced98 (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers174,416 edits Importing Wikidata short description: "Chemical compound"Tag: Shortdesc helper |
| (23 intermediate revisions by 16 users not shown) |
| Line 1: |
Line 1: |
|
|
{{Short description|Chemical compound}} |
|
{{drugbox |
|
|
|
{{Drugbox |
|
| Verifiedfields = changed |
|
|
| UNII_Ref = {{fdacite|changed|FDA}} |
|
| Watchedfields = changed |
|
⚫ |
| verifiedrevid = 443525834 |
| ⚫ |
| UNII = F8252JGO9S |
|
|
⚫ |
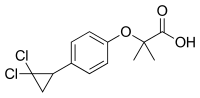
| IUPAC_name = (''RS'')-2--2-<br />methylpropanoic acid |
| ⚫ |
| verifiedrevid = 414072369 |
|
|
| drug_name = Ciprofibrate |
|
| image = Ciprofibrate.svg |
| ⚫ |
| IUPAC_name = (''RS'')-2--2-<br>methylpropanoic acid |
|
|
| image = Ciprofibrate.svg |
|
|
| imagename = 1 : 1 mixture (racemate) |
|
|
| width = 200px |
|
| width = 200px |
|
|
| chirality = ] |
|
| InChI = 1/C13H14Cl2O3/c1-12(2,11(16)17)18-9-5-3-8(4-6-9)10-7-13(10,14)15/h3-6,10H,7H2,1-2H3,(H,16,17) |
|
|
|
<!--Clinical data--> |
| ⚫ |
| smiles = ClC2(Cl)CC2c1ccc(OC(C(=O)O)(C)C)cc1 |
|
|
|
| tradename = |
|
| InChIKey = KPSRODZRAIWAKH-UHFFFAOYAN |
|
|
|
| Drugs.com = {{drugs.com|international|ciprofibrate}} |
|
⚫ |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
|
⚫ |
| pregnancy_category = |
|
⚫ |
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
⚫ |
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
⚫ |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
⚫ |
| legal_status = |
|
⚫ |
| routes_of_administration = |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
⚫ |
| bioavailability = |
|
⚫ |
| protein_bound = |
|
⚫ |
| metabolism = |
|
⚫ |
| elimination_half-life = |
|
⚫ |
| excretion = |
|
|
|
|
|
<!--Identifiers--> |
|
|
| IUPHAR_ligand = 3438 |
|
⚫ |
| CAS_number = 52214-84-3 |
|
⚫ |
| ATC_prefix = C10 |
|
⚫ |
| ATC_suffix = AB08 |
|
⚫ |
| PubChem = 2763 |
|
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
⚫ |
| DrugBank = |
|
⚫ |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
⚫ |
| ChemSpiderID = 2661 |
|
⚫ |
| UNII_Ref = {{fdacite|correct|FDA}} |
|
⚫ |
| UNII = F8252JGO9S |
|
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
⚫ |
| KEGG = D03521 |
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
⚫ |
| ChEBI = 50867 |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL = 557555 |
|
| ChEMBL = 557555 |
|
|
|
|
|
<!--Chemical data--> |
|
⚫ |
| C=13 | H=14 | Cl=2 | O=3 |
|
⚫ |
| smiles = ClC2(Cl)CC2c1ccc(OC(C(=O)O)(C)C)cc1 |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C13H14Cl2O3/c1-12(2,11(16)17)18-9-5-3-8(4-6-9)10-7-13(10,14)15/h3-6,10H,7H2,1-2H3,(H,16,17) |
|
| StdInChI = 1S/C13H14Cl2O3/c1-12(2,11(16)17)18-9-5-3-8(4-6-9)10-7-13(10,14)15/h3-6,10H,7H2,1-2H3,(H,16,17) |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = KPSRODZRAIWAKH-UHFFFAOYSA-N |
|
| StdInChIKey = KPSRODZRAIWAKH-UHFFFAOYSA-N |
| ⚫ |
| CAS_number = 52214-84-3 |
|
| ⚫ |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ⚫ |
| ChemSpiderID = 2661 |
|
| ⚫ |
| ATC_prefix = C10 |
|
| ⚫ |
| ATC_suffix = AB08 |
|
| ⚫ |
| ChEBI = 50867 |
|
| ⚫ |
| PubChem = 2763 |
|
| ⚫ |
| DrugBank = |
|
| ⚫ |
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| ⚫ |
| KEGG = D03521 |
|
| ⚫ |
| C=13|H=14|Cl=2|O=3 |
|
|
| molecular_weight = 289.154 g/mol |
|
| ⚫ |
| bioavailability = |
|
| ⚫ |
| protein_bound = |
|
| ⚫ |
| metabolism = |
|
| ⚫ |
| elimination_half-life = |
|
| ⚫ |
| excretion = |
|
| ⚫ |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| ⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| ⚫ |
| pregnancy_category= |
|
| ⚫ |
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|
| ⚫ |
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
| ⚫ |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
|
| ⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| ⚫ |
| legal_status = |
|
| ⚫ |
| routes_of_administration = |
|
|
}} |
|
}} |
|
|
|
|
'''Ciprofibrate''' is a ]. |
|
'''Ciprofibrate''' is a ] that was developed as a ]. |
|
Crystalline powder white or almost white. |
|
|
|
|
|
Melting point about 115 to 120°C. |
|
|
|
<!-- Society and culture --> |
|
|
It was patented in 1972 and approved for medical use in 1985.<ref name=Fis2006>{{cite book | vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery |date=2006 |publisher=John Wiley & Sons |isbn=9783527607495 |page=474 |url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA474 |language=en}}</ref> |
|
|
|
|
|
==References== |
|
|
{{Reflist|2}} |
|
|
|
|
|
|
|
{{Lipid modifying agents}} |
|
{{Lipid modifying agents}} |
|
|
{{PPAR modulators}} |
|
|
|
|
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
|
|
|
|
|
|
|
|
{{cardiovascular-drug-stub}} |
|
{{cardiovascular-drug-stub}} |
|
|
|
|
] |
|
|
] |
|
|
] |
|
It was patented in 1972 and approved for medical use in 1985.