| Revision as of 07:05, 8 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|errors← Previous edit | Latest revision as of 10:03, 21 October 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,129 edits added Category:4-Hydroxyphenyl compounds using HotCat | ||
| (119 intermediate revisions by 75 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Redirect|DCIP|the children's rights organization|Defence for Children Palestine}} | |||
| {{More citations needed|date=June 2015}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| | verifiedrevid = 443636065 | | verifiedrevid = 443636065 | ||
| | Name = DCPIP | |||
| | ImageFile1 = DCPIP-2D-skeletal.png | | ImageFile1 = DCPIP-2D-skeletal.png | ||
| | ImageSize1 = 150px | | ImageSize1 = 150px | ||
| | ImageFileL2 = Dichlorphenolindophenol-3D-balls.png | | ImageFileL2 = Dichlorphenolindophenol-3D-balls.png | ||
| | ImageSizeL2 = 100px | |||
| | ImageFileR2 = Dichlorphenolindophenol-3D-vdW.png | | ImageFileR2 = Dichlorphenolindophenol-3D-vdW.png | ||
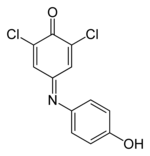
| | PIN = 4-(3,5-dichloro-4-hydroxyphenyl)iminocyclohexa-2,5-dien-1-one | |||
| | ImageSizeR2 = 100px | |||
| ⚫ | | OtherNames = Dichloroindophenol ();<br /> | ||
| | IUPACName = 2,6-dichlorophenol-indophenol | |||
| 2,6-Dichlorophenolindophenol;<br /> | |||
| ⚫ | | |
||
| 2,6-Dichloroindophenol;<br /> | |||
| 2,6- |
2,6-Dichloro-4--2,5-cyclohexadien-1-one | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| 2,6-Dichloro-4-((4-hydroxyphenyl)imino) -2,5-cyclohexadien-1-one | |||
| | Abbreviations = DCPIP, DCIP, DPIP | |||
| ⚫ | | |
||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ⚫ | | ChemSpiderID = 10661857 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C00102 | | KEGG = C00102 | ||
| Line 22: | Line 22: | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 500871 | | ChEMBL = 500871 | ||
| | PubChem = 13726 | |||
| ⚫ | | ChemSpiderID = 10661857 | ||
| | UNII = C35QN2Z58B | |||
| | EC_number = 213-479-8 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C12H7Cl2NO2/c13-10-5-8(6-11(14)12(10)17)15-7-1-3-9(16)4-2-7/h1-6,16H | | StdInChI = 1S/C12H7Cl2NO2/c13-10-5-8(6-11(14)12(10)17)15-7-1-3-9(16)4-2-7/h1-6,16H | ||
| Line 28: | Line 32: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 956-48-9 | | CASNo = 956-48-9 | ||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 945 | | ChEBI = 945 | ||
| | SMILES = Cl\C2=CC(=N/c1ccc(O)cc1)/C=C(/Cl)C2=O | | SMILES = Cl\C2=CC(=N/c1ccc(O)cc1)/C=C(/Cl)C2=O | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | C = 12 | |||
| | Formula = C<sub>12</sub>H<sub>7</sub>NCl<sub>2</sub>O<sub>2</sub> | |||
| | H = 7 | |||
| | MolarMass = 268.1 g mol<sup>−1</sup> | |||
| | |
| N = 1 | ||
| | |
| Cl = 2 | ||
| | |
| O = 2 | ||
| }} | }} | ||
| |Section7 = {{Chembox Hazards | |||
| | GHSPictograms = {{GHS07}} | |||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|302|315|319|335}} | |||
| | PPhrases = {{P-phrases|261|264|270|271|280|301+312|302+352|304+340|305+351+338|312|321|330|332+313|337+313|362|403+233|405|501}} | |||
| }} | |||
| }} | }} | ||
| ⚫ | '''2,6-Dichlorophenolindophenol''' ('''DCPIP''', '''DCIP''' or '''DPIP''') is a ] used as a ]. When ], DCPIP is blue with a maximal absorption at 600 nm; when ], DCPIP is colorless. | ||
| {{cleanup|reason=Needs rewrite to remove unnecessary duplication|date=May 2011}} | |||
| ⚫ | '''2,6- |
||
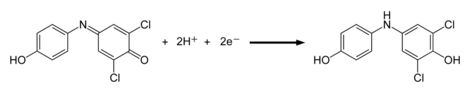
| DCPIP can be used to measure the rate of ]. It is part of the ]s family. When exposed to light in a photosynthetic system, the dye is decolorised by chemical reduction. DCPIP has a higher ] for electrons than ] and the photosynthetic electron transport chain can reduce DCPIP as a substitute for ], that is normally the final electron carrier in photosynthesis. As DCPIP is reduced and becomes colorless, the resultant increase in ] can be measured using a ]. | |||
| ] | ] | ||
| ⚫ | DCPIP can also be used as an indicator for ].<ref>{{cite journal |vauthors=VanderJagt DJ, Garry PJ, Hunt WC |title=Ascorbate in plasma as measured by liquid chromatography and by dichlorophenolindophenol colorimetry |journal=Clin. Chem. |volume=32 |issue=6 |pages=1004–6 |date=June 1986 |pmid=3708799 |url=http://www.clinchem.org/cgi/pmidlookup?view=long&pmid=3708799}}</ref><ref>{{Citation |title=Design an investigation to compare the amount of vitamin C in different fruits and vegetables |url=https://www.youtube.com/watch?v=fxl4Ar2cD0A |access-date=2023-11-18 |language=en}}</ref> If vitamin C, which is a good reducing agent, is present, the blue dye, which turns pink in acid conditions, is reduced to a colorless compound by ascorbic acid. This reaction is a redox reaction: vitamin C (ascorbic acid) is oxidized to ], and DCPIP is reduced to the colorless compound DCPIPH<sub>2</sub> | ||
| ==DCPIP== | |||
| '''DCPIP''' is a ] commonly used as a monitor of the ] in ] because it is an ] acceptor that is blue when ] and colorless when ]. It is part of the ]s family. DCPIP is commonly used as a substitute for ]. The dye changes ] when it is reduced, due to its ]. | |||
| The rate of photosynthesis light-dependent reaction can be measured with this property of DCPIP, because one of the stages of the light reaction is an ] that normally ends with the reduction of NADP<sup>+</sup>. When DCPIP is present, it also gets reduced by the light reaction. The amount of DCPIP reduced can be found by measuring the solution's ] with a ]. | |||
| ⚫ | :DCPIP (blue) + H<sup>+</sup> → DCPIPH (pink) | ||
| ⚫ | DCPIP can also be used as an indicator for ].<ref>{{cite journal | |
||
| ⚫ | :DCPIPH (pink) + vitamin C → DCPIPH<sub>2</sub> (colorless) | ||
| ⚫ | In this ], when all the ascorbic acid in the solution has been used up, there will not be any electrons available to reduce the DCPIPH and the solution remains pink due to the DCPIPH. The end point is a pink color that persists for 10 seconds or more, if there is not enough ascorbic acid to reduce all of the DCPIPH. Pharmacological experiments suggest that DCPIP may serve as a ] chemotherapeutic targeting human cancer cells in an animal model of human ]; DCPIP-induced cancer cell death occurs by depletion of intracellular ] and upregulation of ].<ref>{{cite journal |vauthors=Cabello CM, Bair WB, Bause AS, Wondrak GT |title=Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1 |journal=Biochem. Pharmacol. |volume=78 |issue=4 |pages=344–54 |date=August 2009 |pmid=19394313 |doi=10.1016/j.bcp.2009.04.016 |pmc=2742658}}</ref> | ||
| ⚫ | :DCPIP (blue) + H<sup>+</sup> |
||
| ⚫ | :DCPIPH (pink) + |
||
| :C<sub>6</sub>H<sub>8</sub>O<sub>6</sub> + C<sub>12</sub>H<sub>7</sub>NCl<sub>2</sub>O<sub>2</sub> ——→ C<sub>6</sub>H<sub>6</sub>O<sub>6</sub> + C<sub>12</sub>H<sub>9</sub>NCl<sub>2</sub>O<sub>2</sub> | |||
| ⚫ | In |
||
| ==See also== | ==See also== | ||
| Line 64: | Line 68: | ||
| *] | *] | ||
| *] | *] | ||
| *] | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| *{{cite journal |author=Denby, Derek |journal=Chemistry Review |year=1996 |month=May}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 10:03, 21 October 2024
"DCIP" redirects here. For the children's rights organization, see Defence for Children Palestine.| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Dichlorophenolindophenol" – news · newspapers · books · scholar · JSTOR (June 2015) (Learn how and when to remove this message) |
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 4-(3,5-dichloro-4-hydroxyphenyl)iminocyclohexa-2,5-dien-1-one | |||
| Other names
Dichloroindophenol (); 2,6-Dichlorophenolindophenol; | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Abbreviations | DCPIP, DCIP, DPIP | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.012.254 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C12H7Cl2NO2 | ||
| Molar mass | 268.09 g·mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H315, H319, H335 | ||
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
2,6-Dichlorophenolindophenol (DCPIP, DCIP or DPIP) is a chemical compound used as a redox dye. When oxidized, DCPIP is blue with a maximal absorption at 600 nm; when reduced, DCPIP is colorless.
DCPIP can be used to measure the rate of photosynthesis. It is part of the Hill reagents family. When exposed to light in a photosynthetic system, the dye is decolorised by chemical reduction. DCPIP has a higher affinity for electrons than ferredoxin and the photosynthetic electron transport chain can reduce DCPIP as a substitute for NADP, that is normally the final electron carrier in photosynthesis. As DCPIP is reduced and becomes colorless, the resultant increase in light transmittance can be measured using a spectrophotometer.
DCPIP can also be used as an indicator for vitamin C. If vitamin C, which is a good reducing agent, is present, the blue dye, which turns pink in acid conditions, is reduced to a colorless compound by ascorbic acid. This reaction is a redox reaction: vitamin C (ascorbic acid) is oxidized to dehydroascorbic acid, and DCPIP is reduced to the colorless compound DCPIPH2
- DCPIP (blue) + H → DCPIPH (pink)
- DCPIPH (pink) + vitamin C → DCPIPH2 (colorless)
In this titration, when all the ascorbic acid in the solution has been used up, there will not be any electrons available to reduce the DCPIPH and the solution remains pink due to the DCPIPH. The end point is a pink color that persists for 10 seconds or more, if there is not enough ascorbic acid to reduce all of the DCPIPH. Pharmacological experiments suggest that DCPIP may serve as a pro-oxidant chemotherapeutic targeting human cancer cells in an animal model of human melanoma; DCPIP-induced cancer cell death occurs by depletion of intracellular glutathione and upregulation of oxidative stress.
See also
References
- VanderJagt DJ, Garry PJ, Hunt WC (June 1986). "Ascorbate in plasma as measured by liquid chromatography and by dichlorophenolindophenol colorimetry". Clin. Chem. 32 (6): 1004–6. PMID 3708799.
- Design an investigation to compare the amount of vitamin C in different fruits and vegetables, retrieved 2023-11-18
- Cabello CM, Bair WB, Bause AS, Wondrak GT (August 2009). "Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1". Biochem. Pharmacol. 78 (4): 344–54. doi:10.1016/j.bcp.2009.04.016. PMC 2742658. PMID 19394313.