| Revision as of 08:50, 9 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').← Previous edit | Latest revision as of 01:26, 3 December 2023 edit undo213.230.114.155 (talk) Last name has to be capitalized | ||
| (34 intermediate revisions by 23 users not shown) | |||
| Line 1: | Line 1: | ||
| {{unreferenced|date=June 2010}} | |||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 443837963 | ||
| | ImageFile = Glucose 1-phosphate.svg | | ImageFile = Glucose 1-phosphate.svg | ||
| | ImageSize = 150px | | ImageSize = 150px | ||
| | ImageCaption = Anionic form of α-<small>D</small>-glucose 1-phosphate | |||
| | ImageFile2 = |
| ImageFile2 =Cori_ester.png | ||
| | ImageSize2 = 150px | | ImageSize2 = 150px | ||
| | |
| ImageCaption2 = Neutral form of α-<small>D</small>-glucose 1-phosphate | ||
| | IUPACName = <small>D</small>-Glucopyranosyl dihydrogen phosphate | |||
| ⚫ | | OtherNames = | ||
| | SystematicName = (2''Ξ'',3''R'',4''S'',5''S'',6''R'')-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl dihydrogen phosphate | |||
| ⚫ | | |
||
| ⚫ | | OtherNames = Cori ester | ||
| ⚫ | | |
||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 388311 | | ChemSpiderID = 388311 | ||
| | InChI = 1/C6H13O9P/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13/h2-10H,1H2,(H2,11,12,13)/t2-,3-,4+,5-,6?/m1/s1 | | InChI = 1/C6H13O9P/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13/h2-10H,1H2,(H2,11,12,13)/t2-,3-,4+,5-,6?/m1/s1 | ||
| Line 20: | Line 22: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 59-56-3 | | CASNo = 59-56-3 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | |
| UNII = CIX3U01VAU | ||
| ⚫ | | PubChem = 65533 | ||
| | KEGG = C00103 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 16077 | |||
| | SMILES = O=P(O)(OC1O((O)(O)1O)CO)O | | SMILES = O=P(O)(OC1O((O)(O)1O)CO)O | ||
| | |
| MeSHName = glucose-1-phosphate | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | C=6 | H=13 | O=9 | P=1 | |||
| | Formula = C6H13O9P | |||
| | |
| Appearance = | ||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| | BoilingPt = | |||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | Autoignition = | |||
| }} | }} | ||
| }} | }} | ||
| ⚫ | '''Glucose 1-phosphate''' (also called ''' |
||
| ⚫ | '''Glucose 1-phosphate''' (also called '''Cori ester''') is a ] molecule with a ] group on the 1'-carbon. It can exist in either the α- or β-]ic form. | ||
| ==Reactions== | |||
| ==Reactions of α-glucose 1-phosphate== | |||
| ===Catabolic=== | ===Catabolic=== | ||
| In ], it is the direct product of the reaction in which ] cleaves off a ] of ] from a greater ] structure. | In ], it is the direct product of the reaction in which ] cleaves off a ] of ] from a greater ] structure. A deficiency of muscle glycogen phosphorylase is known as ] (McArdle Disease). | ||
| To be utilized in cellular catabolism it must first be converted to ] by the ] ] in a free equilibrium.<ref>{{Citation|last=Pelley|first=John W.|title=8 - Gluconeogenesis and Glycogen Metabolism|date=2012-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780323074469000088|work=Elsevier's Integrated Review Biochemistry (Second Edition)|pages=67–73|editor-last=Pelley|editor-first=John W.|place=Philadelphia|publisher=W.B. Saunders|language=en|doi=10.1016/b978-0-323-07446-9.00008-8|isbn=978-0-323-07446-9|access-date=2020-12-16}}</ref><ref>{{Citation|last=Isselbacher|first=Kurt J.|title=Galactose-1-phosphate Uridyl Transferase|date=1965-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780123956309501535|work=Methods of Enzymatic Analysis|pages=863–866|editor-last=Bergmeyer|editor-first=Hans-Ulrich|publisher=Academic Press|language=en|doi=10.1016/b978-0-12-395630-9.50153-5|isbn=978-0-12-395630-9|access-date=2020-12-16}}</ref><ref>{{Citation|last=Bergmeyer|first=Hans-Ulrich|title=d-Glucose-1-phosphate|date=1965-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780123956309500244|work=Methods of Enzymatic Analysis|pages=131–133|editor-last=Bergmeyer|editor-first=Hans-Ulrich|publisher=Academic Press|language=en|doi=10.1016/b978-0-12-395630-9.50024-4|isbn=978-0-12-395630-9|access-date=2020-12-16|last2=Klotzsch|first2=Helmut}}</ref> One reason that cells form glucose 1-phosphate instead of glucose during glycogen breakdown is that the very polar phosphorylated glucose cannot leave the cell membrane and so is marked for intracellular catabolism. ] deficiency is known as ] type 14 (GSD XIV).<ref></ref> | |||
| To be utilized in cellular catabolism it must first be converted to ] by the ] ]. One reason that cells form glucose 1-phosphate instead of glucose during glycogen breakdown is that the very polar phosphorylated glucose cannot leave the cell membrane and so is marked for intracellular catabolism. | |||
| ===Anabolic=== | ===Anabolic=== | ||
| In ], free glucose 1-phosphate can also react with ] to form ], by using the enzyme ]. It can then return to the greater glycogen structure via ]. | In ], free glucose 1-phosphate can also react with ] to form ],<ref name=":0">{{Citation|last=Blanco|first=Antonio|title=Chapter 19 - Integration and Regulation of Metabolism|date=2017-01-01|url=http://www.sciencedirect.com/science/article/pii/B9780128035504000197|work=Medical Biochemistry|pages=425–445|editor-last=Blanco|editor-first=Antonio|publisher=Academic Press|language=en|doi=10.1016/b978-0-12-803550-4.00019-7|isbn=978-0-12-803550-4|access-date=2020-12-16|last2=Blanco|first2=Gustavo|editor2-last=Blanco|editor2-first=Gustavo}}</ref> by using the enzyme ]. It can then return to the greater glycogen structure via ].<ref name=":0" /> | ||
| ==β-Glucose 1-phosphate== | |||
| β-Glucose 1-phosphate is found in some microbes. It is produced by inverting α-glucan phosphorylases including ], ] and ] and is then converted into ] by ]. | |||
| ==See also== | ==See also== | ||
| * ] | * ] | ||
| * ] | |||
| ==References== | |||
| ⚫ | ] | ||
| {{reflist}} | |||
| ⚫ | ] | ||
| {{biochem-stub}} | |||
| {{Glycogenesis and glycogenolysis metabolic intermediates}} | {{Glycogenesis and glycogenolysis metabolic intermediates}} | ||
| {{Fructose and galactose metabolic intermediates}} | {{Fructose and galactose metabolic intermediates}} | ||
| ⚫ | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 01:26, 3 December 2023
 Anionic form of α-D-glucose 1-phosphate | |
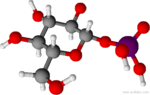 Neutral form of α-D-glucose 1-phosphate | |
| Names | |
|---|---|
| IUPAC name D-Glucopyranosyl dihydrogen phosphate | |
| Systematic IUPAC name (2Ξ,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl dihydrogen phosphate | |
| Other names Cori ester | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.396 |
| KEGG | |
| MeSH | glucose-1-phosphate |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H13O9P |
| Molar mass | 260.135 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Glucose 1-phosphate (also called Cori ester) is a glucose molecule with a phosphate group on the 1'-carbon. It can exist in either the α- or β-anomeric form.
Reactions of α-glucose 1-phosphate
Catabolic
In glycogenolysis, it is the direct product of the reaction in which glycogen phosphorylase cleaves off a molecule of glucose from a greater glycogen structure. A deficiency of muscle glycogen phosphorylase is known as glycogen storage disease type V (McArdle Disease).
To be utilized in cellular catabolism it must first be converted to glucose 6-phosphate by the enzyme phosphoglucomutase in a free equilibrium. One reason that cells form glucose 1-phosphate instead of glucose during glycogen breakdown is that the very polar phosphorylated glucose cannot leave the cell membrane and so is marked for intracellular catabolism. Phosphoglucomutase-1 deficiency is known as glycogen storage disease type 14 (GSD XIV).
Anabolic
In glycogenesis, free glucose 1-phosphate can also react with UTP to form UDP-glucose, by using the enzyme UDP-glucose pyrophosphorylase. It can then return to the greater glycogen structure via glycogen synthase.
β-Glucose 1-phosphate
β-Glucose 1-phosphate is found in some microbes. It is produced by inverting α-glucan phosphorylases including maltose phosphorylase, kojibiose phosphorylase and trehalose phosphorylase and is then converted into glucose 6-phosphate by β-phosphoglucomutase.
See also
References
- Pelley, John W. (2012-01-01), Pelley, John W. (ed.), "8 - Gluconeogenesis and Glycogen Metabolism", Elsevier's Integrated Review Biochemistry (Second Edition), Philadelphia: W.B. Saunders, pp. 67–73, doi:10.1016/b978-0-323-07446-9.00008-8, ISBN 978-0-323-07446-9, retrieved 2020-12-16
- Isselbacher, Kurt J. (1965-01-01), Bergmeyer, Hans-Ulrich (ed.), "Galactose-1-phosphate Uridyl Transferase", Methods of Enzymatic Analysis, Academic Press, pp. 863–866, doi:10.1016/b978-0-12-395630-9.50153-5, ISBN 978-0-12-395630-9, retrieved 2020-12-16
- Bergmeyer, Hans-Ulrich; Klotzsch, Helmut (1965-01-01), Bergmeyer, Hans-Ulrich (ed.), "d-Glucose-1-phosphate", Methods of Enzymatic Analysis, Academic Press, pp. 131–133, doi:10.1016/b978-0-12-395630-9.50024-4, ISBN 978-0-12-395630-9, retrieved 2020-12-16
- Orphanet: Glycogen storage disease due to phosphoglucomutase deficiency
- ^ Blanco, Antonio; Blanco, Gustavo (2017-01-01), Blanco, Antonio; Blanco, Gustavo (eds.), "Chapter 19 - Integration and Regulation of Metabolism", Medical Biochemistry, Academic Press, pp. 425–445, doi:10.1016/b978-0-12-803550-4.00019-7, ISBN 978-0-12-803550-4, retrieved 2020-12-16
| Glycogenesis and glycogenolysis metabolic intermediates | |
|---|---|
| Glucose | |
| Uridine | |
| Other | |
| Fructose and galactose metabolic intermediates | |||||||
|---|---|---|---|---|---|---|---|
| Fructose | |||||||
| Galactose | |||||||
| Mannose | |||||||