| Revision as of 03:10, 10 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validation|Chem/Drugbox← Previous edit | Latest revision as of 22:49, 4 April 2023 edit undoEntranced98 (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers174,416 edits Importing Wikidata short description: "Chemical compound"Tag: Shortdesc helper | ||
| (21 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{drugbox | |||
| {{Drugbox | |||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 443987359 | ||
| ⚫ | | IUPAC_name = 1-Pyridin-4-yl-''N''-(pyridin-4-ylmethyl)methanamine | ||
| ⚫ | | image = Gapicomine.png | ||
| <!--Clinical data--> | |||
| | tradename = | |||
| ⚫ | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| ⚫ | | pregnancy_category = | ||
| ⚫ | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | ||
| ⚫ | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| ⚫ | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| ⚫ | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| ⚫ | | legal_status = off market (was used in EU countries) | ||
| ⚫ | | routes_of_administration = ] (tablet) | ||
| <!--Pharmacokinetic data--> | |||
| ⚫ | | bioavailability = | ||
| ⚫ | | protein_bound = | ||
| ⚫ | | metabolism = | ||
| ⚫ | | elimination_half-life = | ||
| ⚫ | | excretion = | ||
| <!--Identifiers--> | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| ⚫ | | ATC_prefix = none | ||
| ⚫ | | ATC_suffix = | ||
| ⚫ | | PubChem = 68955 | ||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| ⚫ | | DrugBank = | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = WWW0P95393 | | UNII = WWW0P95393 | ||
| ⚫ | | CAS_number = 1539-39-5 | ||
| ⚫ | | verifiedrevid = |
||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ⚫ | | IUPAC_name |
||
| | ChEMBL = 2103958 | |||
| ⚫ | | image |
||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ⚫ | | CAS_number |
||
| | ChemSpiderID = 62178 | |||
| ⚫ | | ATC_prefix |
||
| | smiles = n1ccc(cc1)CNCc2ccncc2 | |||
| ⚫ | | ATC_suffix |
||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | PubChem |
||
| | StdInChI = 1S/C12H13N3/c1-5-13-6-2-11(1)9-15-10-12-3-7-14-8-4-12/h1-8,15H,9-10H2 | |||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | DrugBank |
||
| | StdInChIKey = AUQQZPGNRKTPSQ-UHFFFAOYSA-N | |||
| ⚫ | | C=12|H=13|N=3 | ||
| <!--Chemical data--> | |||
| | molecular_weight = 199.25 g/mol | |||
| ⚫ | | C=12 | H=13 | N=3 | ||
| ⚫ | | bioavailability |
||
| ⚫ | | protein_bound |
||
| ⚫ | | metabolism |
||
| ⚫ | | elimination_half-life = | ||
| ⚫ | | excretion |
||
| ⚫ | | pregnancy_AU |
||
| | pregnancy_US = <!-- A / B / C / D / X --> | |||
| ⚫ | | pregnancy_category |
||
| ⚫ | | legal_AU |
||
| ⚫ | | legal_CA |
||
| ⚫ | | legal_UK |
||
| ⚫ | | legal_US |
||
| ⚫ | | legal_status |
||
| ⚫ | | routes_of_administration = |
||
| }} | }} | ||
| '''Gapicomine''' (]) is a ] ]. It has been withdrawn from the market in the countries it was used in.<ref>{{cite web |url=http://www.justscience.de/en/drugbase/indexnominum/monograph.html?tx_crondavindexnominum_pi%5Bmonograph_uid%5D=3597&cHash=e51fae0af1 |title=Gapicomine Monograph, The Index Nominum | |
'''Gapicomine''' (]) is a ] ]. It has been withdrawn from the market in the countries it was used in.<ref>{{cite web |url=http://www.justscience.de/en/drugbase/indexnominum/monograph.html?tx_crondavindexnominum_pi%5Bmonograph_uid%5D=3597&cHash=e51fae0af1 |title=Gapicomine Monograph, The Index Nominum |access-date=2008-03-31 }}</ref> | ||
| Also, gapicomine is a major component in the drug ].<ref>{{cite web |url= |
Also, gapicomine is a major component in the drug ].<ref>{{cite web |url=https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=211061 |title=Bicordin, PubChem |access-date=2008-03-31 }}</ref> | ||
| ==History== | ==History== | ||
| Gapicomine was discovered in 1970 by ] ] ]. It was first published about in an article of ''The Polish Journal of Medicine and Pharmacy'' describing the derivative drug ] in 1974.<ref name="PMID4453155">{{cite journal | |
Gapicomine was discovered in 1970 by ] ] ]. It was first published about in an article of ''The Polish Journal of Medicine and Pharmacy'' describing the derivative drug ] in 1974.<ref name="PMID4453155">{{cite journal |vauthors=Samochowiec L, Wójcicki J, Gregorczyk K, Szmatloch E |title=Bicordin--a new drug in the treatment of coronary heart disease. |journal=Mater Med Pol. |volume= 6|issue= 4|pages=298–300 |year=1974 |pmid=4453155 }}</ref> | ||
| ==Synthesis== | |||
| ] | |||
| The oxime formation between isonicotinaldehyde ('''1''') and hydroxylamine gives 4-Pyridinealdoxime ('''2'''). This is then reduced by catalytic hydrogenation over Raney-Nickel into 4-Picolylamine ('''3'''). Reductive amination of the last with a second equivalent of isonicotinaldehyde affords gapicomine ('''4'''). | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Vasodilators used in cardiac diseases}} | {{Vasodilators used in cardiac diseases}} | ||
| ] | ] | ||
| ] | ] | ||
| {{cardiovascular-drug-stub}} | {{cardiovascular-drug-stub}} | ||
| ] | |||
Latest revision as of 22:49, 4 April 2023
Chemical compound Pharmaceutical compound | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth (tablet) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H13N3 |
| Molar mass | 199.257 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Gapicomine (INN) is a coronary vasodilator. It has been withdrawn from the market in the countries it was used in.
Also, gapicomine is a major component in the drug Bicordin.
History
Gapicomine was discovered in 1970 by Polish chemist Stanisław Biniecki. It was first published about in an article of The Polish Journal of Medicine and Pharmacy describing the derivative drug Bicordin in 1974.
Synthesis
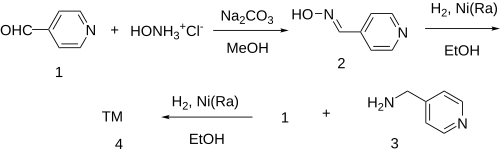
The oxime formation between isonicotinaldehyde (1) and hydroxylamine gives 4-Pyridinealdoxime (2). This is then reduced by catalytic hydrogenation over Raney-Nickel into 4-Picolylamine (3). Reductive amination of the last with a second equivalent of isonicotinaldehyde affords gapicomine (4).
References
- "Gapicomine Monograph, The Index Nominum". Retrieved 2008-03-31.
- "Bicordin, PubChem". Retrieved 2008-03-31.
- Samochowiec L, Wójcicki J, Gregorczyk K, Szmatloch E (1974). "Bicordin--a new drug in the treatment of coronary heart disease". Mater Med Pol. 6 (4): 298–300. PMID 4453155.
- Anon., GB 1058356 (1967 to Starogardzkie Zakl Farma).
| Vasodilators used in cardiac diseases (C01D) | |
|---|---|
| Nitrovasodilators | |
| Quinolone vasodilators | |
| Others | |
| |
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |