| Revision as of 11:32, 10 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to verified fields - updated 'DrugBank_Ref', 'UNII_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBot|← Previous edit | Latest revision as of 01:20, 18 May 2024 edit undoBeland (talk | contribs)Autopatrolled, Administrators237,084 editsm change U+00B5 to U+03BC (μ) per Unicode standard and MOS:NUM#Specific units - see Unicode compatibility characters (via WP:JWB) | ||
| (94 intermediate revisions by 60 users not shown) | |||
| Line 1: | Line 1: | ||
| {{redirect|Claviform|the botany and zoology term|Glossary of entomology terms#clavate}} | |||
| {{chembox | |||
| {{Chembox | |||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 408789487 | |||
| | verifiedrevid = 462273608 | |||
| | Reference = <ref name="Merck"/> | |||
| | Reference = <ref name="merck">Merck Index, 11th Edition, 7002</ref> | |||
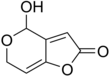
| | ImageFileL1 = Patulin.png | | ImageFileL1 = Patulin.png | ||
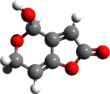
| | ImageFileR1 = Patulin_3d_structure.png | |||
| | ImageSizeL1 = 120px | |||
| | ImageFileR1=Patulin_3d_structure.png | |||
| | ImageSizeR2 = 120px | |||
| | IUPACName = 4-hydroxy-4''H''-furopyran-2(6''H'')-one | | IUPACName = 4-hydroxy-4''H''-furopyran-2(6''H'')-one | ||
| | OtherNames = 2-Hydroxy-3,7-dioxabicyclonona-5,9-dien-8-one<br /> | | OtherNames = 2-Hydroxy-3,7-dioxabicyclonona-5,9-dien-8-one<br /> | ||
| Clairformin<br />Claviform<br />Expansine<br />Clavacin<br />Clavatin<br />Expansin<br />Gigantin<br />Leucopin<br />Patuline | Clairformin<br />Claviform<br />Expansine<br />Clavacin<br />Clavatin<br />Expansin<br />Gigantin<br />Leucopin<br />Patuline | ||
| | |
|Section1={{Chembox Identifiers | ||
| | Abbreviations = | | Abbreviations = | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| Line 28: | Line 28: | ||
| | KEGG_Ref = {{keggcite|changed|kegg}} | | KEGG_Ref = {{keggcite|changed|kegg}} | ||
| | KEGG = C16748 | | KEGG = C16748 | ||
| | UNII_Ref = {{fdacite| |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 95X2BV4W8R | | UNII = 95X2BV4W8R | ||
| | SMILES = O=C\1O/C2=C/COC(O)C2=C/1 | | SMILES = O=C\1O/C2=C/COC(O)C2=C/1 | ||
| | InChI = | |||
| | RTECS = | | RTECS = | ||
| | MeSHName = | | MeSHName = | ||
| | ChEBI_Ref = {{ebicite| |
| ChEBI_Ref = {{ebicite|changed|EBI}} | ||
| | ChEBI = | | ChEBI = 74926 | ||
| }} | |||
| | ATCCode_prefix = | |||
| |Section2={{Chembox Properties | |||
| | ATCCode_suffix = | |||
| | C=7 | H=6 | O=4 | |||
| | ATC_Supplemental =}} | |||
| | Section2 = {{Chembox Properties | |||
| | C=7|H=6|O=4 | |||
| | MolarMass = 154.12 g/mol | | MolarMass = 154.12 g/mol | ||
| | Appearance = Compact prisms | | Appearance = Compact prisms | ||
| | Density = | | Density = 1.52 g/mL | ||
| | MeltingPtC = 110 | | MeltingPtC = 110 | ||
| | |
| MeltingPt_notes = | ||
| | |
| BoilingPtC = | ||
| | |
| BoilingPt_notes = | ||
| | Solubility = Soluble | | Solubility = Soluble | ||
| | SolubleOther = | | SolubleOther = | ||
| | Solvent = | | Solvent = | ||
| | pKa = | | pKa = | ||
| | pKb = }} | | pKb = | ||
| }} | |||
| | |
|Section7={{Chembox Hazards | ||
| | EUClass = | |||
| | EUIndex = | |||
| | MainHazards = | | MainHazards = | ||
| | NFPA-H = | | NFPA-H = | ||
| | NFPA-F = | | NFPA-F = | ||
| | NFPA-R = | | NFPA-R = | ||
| | NFPA- |
| NFPA-S = | ||
| | |
| HPhrases = | ||
| | |
| PPhrases = | ||
| | |
| GHS_ref = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | ExploLimits = | | ExploLimits = | ||
| | PEL = }} | | PEL = | ||
| }} | |||
| }} | }} | ||
| '''Patulin''' is an ] classified as a ]. It is named after the ] from which it was isolated, '']''. It is a white powder soluble in acidic water and in ]s. It is a ] that is heat-stable, so it is not destroyed by ] or thermal ].<ref name="sigma"> sigmaaldrich.com</ref> However, stability following ] is lessened.<ref name="ucm212520">{{cite web |url=https://www.fda.gov/food/foodborneillnesscontaminants/naturaltoxins/ucm212520.htm |title=Patulin in Apple Juice, Apple Juice Concentrates and Apple Juice Products |website=www.fda.gov |url-status=dead |archive-url=https://web.archive.org/web/20130815072813/http://www.fda.gov/Food/FoodborneIllnessContaminants/NaturalToxins/ucm212520.htm |archive-date=2013-08-15}}</ref> It is a ] produced by a variety of molds, in particular, '']'' and '']'' and '']''. Most commonly found in rotting ], the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. Its presence is highly regulated. | |||
| '''Patulin''' is a ] produced by a variety of molds, in particular, '']'' and '']''. It is commonly found in rotting apples, and the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. It is not a particularly potent toxin, but a number of studies have shown that it is ], which has led to some theories that claim that it may be a carcinogen, though animal studies have remained inconclusive.<ref>{{cite journal | |||
| | authorlink = Ellen C. Hopmans | |||
| | title = Patulin: a Mycotoxin in Apples | |||
| | journal = Perishables Handling Quarterly | |||
| | issue = 91 | |||
| | date = August 1997 | |||
| | url = http://ucce.ucdavis.edu/files/datastore/234-166.pdf | |||
| | pages = 5 }}</ref> Patulin is also an antibiotic.<ref name="Merck">''Merck Index'', 11th Edition, '''7002'''.</ref> Several countries have instituted patulin restrictions in apple products. The ] recommends a maximum concentration of 50 µg/L in apple juice.<ref>{{cite web | |||
| | title = Foodborne hazards (World Health Organization | |||
| | url=http://www.who.int/foodsafety/publications/capacity/en/2.pdf | |||
| | accessdate = 2007-01-22 }}</ref> | |||
| ==Biosynthesis, synthesis, and reactivity== | |||
| In European Union, the limit is set to 50 micrograms per kilogram (µg/kg) in both apple juice and cider, and to half of that concentration, 25 µg/kg in solid apple products and 10 µg/kg in products for infants and young children. These limits came into force on 1 November 2003. <ref> information leaf from ]</ref> | |||
| Patulin is biosynthesized from ] via multiple chemical transformations.<ref>{{cite journal | doi = 10.3390/toxins2040613 | doi-access = free | title = Biosynthesis and Toxicological Effects of Patulin | date = 2010 | last1 = Puel | first1 = Olivier | last2 = Galtier | first2 = Pierre | last3 = Oswald | first3 = Isabelle | journal = Toxins | volume = 2 | issue = 4 | pages = 613–631 | pmid = 22069602 | pmc = 3153204 }}</ref> | |||
| Isoepoxydon dehydrogenase (IDH) is an important ] in the multi-step biosynthesis of patulin. Its gene is present in other fungi that may potentially produce the toxin.<ref name="Puel">{{cite journal|last1=Puel|first1=Olivier|last2=Galtier|first2=Pierre|last3 =Oswald|first3=Isabelle P.|title=Biosynthesis and Toxicological Effects of Patulin|journal=Toxins|date=5 April 2010|volume=2|issue=4|pages=613–631|doi=10.3390/toxins2040613|pmid=22069602|pmc=3153204|doi-access=free}}</ref> It is reactive with ], so antioxidant and antimicrobial agents may be useful to destroy it.<ref name="Llewellyn">{{Cite journal | doi = 10.1016/s0278-6915(98)00084-2 | title = Immunological evaluation of the mycotoxin patulin in female b6C3F1 mice | date = 1998 | last1 = Llewellyn | first1 = G.C | last2 = McCay | first2 = J.A | last3 = Brown | first3 = R.D | last4 = Musgrove | first4 = D.L | last5 = Butterworth | first5 = L.F | last6 = Munson | first6 = A.E | last7 = White | first7 = K.L | journal = Food and Chemical Toxicology | volume = 36 | issue = 12 | pages = 1107–1115 | pmid = 9862653 }}</ref> Levels of nitrogen, manganese, and pH as well as abundance of necessary enzymes regulate the biosynthetic pathway of patulin.<ref name="Puel" /> | |||
| == References == | |||
| == Uses == | |||
| <references/> | |||
| Patulin was originally used as an antibiotic against Gram-positive and Gram-negative bacteria, but after several toxicity reports, it is no longer used for that purpose.<ref name="MRC">Medical Research Council. Clinical trial of patulin in the common cold. ''Lancet''1944; ii: 373-5.</ref> Isolated by ] in 1943, it was specifically trialed to be used against the common cold.<ref name="MRC" /> Patulin is used as a potassium-uptake inhibitor in laboratory applications.<ref name="sigma" /> Kashif Jilani and co-workers reported that patulin stimulates suicidal ] death under physiological concentrations.<ref>{{cite journal|last=Lupescu|first=A|author2=Jilani, K |author3=Zbidah, M |author4= Lang, F |title=Patulin-induced suicidal erythrocyte death.|journal=Cellular Physiology and Biochemistry|date=2013|volume=32|issue=2|pages=291–9|pmid=23942252 |doi=10.1159/000354437|doi-access=free}}</ref> | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| == Sources of exposure == | |||
| ] | |||
| Frequently, patulin is found in apples and apple products such as juices, jams, and ciders. It has also been detected in other fruits including cherries, blueberries, plums, bananas, strawberries, and grapes.<ref name="Llewellyn" /> Fungal growth leading to patulin production is most common on damaged fruits.<ref name="pippin">{{cite web |url=http://www.orangepippin.com/resources/general/patulin |title=Patulin |access-date=2013-11-25 |url-status=dead |archive-url=https://web.archive.org/web/20131018034231/http://www.orangepippin.com/resources/general/patulin |archive-date=2013-10-18 }}</ref> Patulin has also been detected in grains like barley, wheat, corn and their processed products as well as in shellfish.<ref name="Llewellyn" /><ref name="Pouchous">Pouchous et al. Shellfish</ref>{{full|date=January 2024}} | |||
| ] | |||
| Dietary intake of patulin from apple juice has been estimated at between 0.03 and 0.26 μg per kg body weight per day in various age groups and populations.<ref name="wouters">Wouters, FA, and Speijers, GJA. JECFA Monograph on Patulin. World Health Organization Food Additives Series 35 (http://www.inchem.org/documents/jecfa/jecmono/v26je10.htm)</ref> Content of patulin in apple juice is estimated to be less than 10–15 μg/L.<ref name="wouters" /> A number of studies have looked into comparisons of organic vs conventional harvest of apples and levels of patulin contamination.<ref>Pique, E., et al. Occurrence of patulin in organic and conventional apple juice. Risk Assessment. Recent Advances in Pharmaceutical Sciences, III, 2013: 131–144.</ref><ref>{{Cite journal|last1=Piemontese|first1=L.|last2=Solfrizzo|first2=M.|last3=Visconti|first3=A.|date=2005-05-01|title=Occurrence of patulin in conventional and organic fruit products in Italy and subsequent exposure assessment|journal=Food Additives and Contaminants|volume=22|issue=5|pages=437–442|doi=10.1080/02652030500073550|issn=0265-203X|pmid=16019815|s2cid=31155096}}</ref><ref>{{cite journal|last1=Piqué|first1=E|last2=Vargas-Murga|first2=L|last3=Gómez-Catalán|first3=J|last4=Lapuente|first4=Jd|last5=Llobet|first5=JM|title=Occurrence of patulin in organic and conventional apple-based food marketed in Catalonia and exposure assessment.|journal=Food and Chemical Toxicology |date=October 2013|volume=60|pages=199–204|doi=10.1016/j.fct.2013.07.052|pmid=23900007}}</ref> For example, one study showed 0.9% of children drinking organic apple juice exceeded the ] (TDI) for patulin.<ref>Beark et al 2007</ref>{{full|date=January 2024}} A recent article described detection of patulin in marine strains of Penicillium, indicating a potential risk in shellfish consumption.<ref name="Pouchous" /> | |||
| ] | |||
| ] | |||
| == Toxicity == | |||
| ] | |||
| A subacute rodent ] of 43 μg/kg body weight as well as ] studies were primarily the cause for setting limits for patulin exposure, although a range of other types of toxicity also exist.<ref name="ucm212520" /> | |||
| ] | |||
| ] | |||
| While not a particularly potent toxin, patulin is ]. Some theorize that it may be a carcinogen, although animal studies have remained inconclusive.<ref>"Patulin: a Mycotoxin in Apples". Perishables Handling Quarterly (91): 5. August 1997</ref> Patulin has shown antimicrobial properties against some microorganisms.<ref name="merck" /> Several countries have instituted patulin restrictions in apple products. The ] recommends a maximum concentration of 50 μg/L in apple juice.<ref name="FHWHO">"Foodborne hazards (World Health Organization". Retrieved 2007-01-22.</ref> In the European Union, the limit is also set at 50 micrograms per kilogram (μg/kg) in apple juice and cider, at 25 μg/kg in solid apple products, and at 10 μg/kg in products for infants and young children. These limits came into force on 1 November 2003.<ref>Patulin information leaf from Fermentek</ref>{{full|date=January 2024}} | |||
| ] | |||
| ] | |||
| === Acute === | |||
| ] | |||
| Patulin is toxic primarily through affinity to sulfhydryl groups (SH), which results in inhibition of enzymes. Oral ] in rodent models have ranged between 20 and 100 mg/kg.<ref name="ucm212520" /> In poultry, the oral LD<sub>50</sub> range was reported between 50 and 170 mg/kg.<ref name="Puel" /> Other routes of exposure are more toxic, yet less likely to occur. Major acute toxicity findings include gastrointestinal problems, ] (i.e. convulsions), ], and ].<ref name="ucm212520" /> | |||
| ] | |||
| ] | |||
| === Subacute === | |||
| ] | |||
| Studies in rats showed decreased weight, and gastric, intestinal, and renal function changes, while repetitive doses lead to neurotoxicity. Reproductive toxicity in males was also reported.<ref name="Puel" /> A ] in rodents was observed at 43 μg/kg body weight.<ref name="ucm212520" /> | |||
| === Genotoxicity === | |||
| ] concluded that patulin is genotoxic based on variable ] data, however it is considered a group 3 carcinogen by the ] (IARC) since data was inconclusive.<ref name="ucm212520" /> | |||
| === Reproduction studies === | |||
| Patulin decreased sperm count and altered sperm morphology in the rat.<ref>{{cite journal | last1 = Selmanoglu | first1 = G | title = Evaluation of the reproductive toxicity of patulin in growing male rats | doi = 10.1016/j.fct.2006.06.022 | journal = Food Chem. Toxicol. | year = 2006 | volume = 44| issue = 12 | pages = 2019–2024 | pmid = 16905234 }}</ref> | |||
| Also, it resulted in abortion of ] litters in rats and mice after i.p. injection.<ref name="Puel" /> Embryotoxicity and ] were also reported in chick eggs.<ref name="Puel" /> | |||
| === Immunotoxicity === | |||
| Patulin was found to be ] in a number of animal and even human studies. Reduced cytokine secretion, oxidative burst in macrophages, increased splenic ], and increased ] numbers are a few endpoints noticed.<ref name="Puel" /> However, dietary relevant exposure would not be likely to alter immune response.<ref name="Llewellyn" /> | |||
| === Human health === | |||
| Although there are only very few reported cases and epidemiological data, the ] has set an action limit of 50 ppb in cider due to its potential ] and other reported adverse effects.<ref name="ucm212520" /> | |||
| In humans, it was tested as an ] intranasally for use against the ] with few significant ]s, yet also had negligible or no beneficial effect.<ref name="MRC" /> | |||
| == Risk management and regulations == | |||
| Patulin exposure can be successfully managed by following ] such as removing mold, washing, and not using rotten or damaged apples for baking, canning, or juice production.<ref name="ucm212520" /><ref name="pippin" /> | |||
| '''US''' | |||
| The provisional ] (PTDI) for patulin was set at 0.43 μg/kg body weight by the FDA<ref name="ucm212520" /> based on a ] of 0.3 mg/kg body weight per week.<ref name="ucm212520" /> ] was done on apple juice to compare exposure and the PTDI. Without controls or an action limit, the 90th percentile of consumers would not be above the PTDI. However, the concentration in children 1–2 years old would be three times as high as the PDTI, hence an action limit of 50 μg/kg.<ref name="ucm212520" /> | |||
| '''WHO''' | |||
| The ] recommends a maximum concentration of 50 μg/L in apple juice.<ref name="FHWHO" /> | |||
| '''EU''' | |||
| The ] (EU) has set a maximum limit of 50 μg/kg on fruit juices and drinks, while solid apple products have a limit of 25 μg/kg. For certain foods intended for infants, an even lower limit of 10 μg/kg is observed. | |||
| To test for patulin contamination, a variety of methods and sample preparation methods have been employed, including ] (TLC), ] (GC), ] (HPLC), and ].<ref>{{cite journal | doi = 10.1016/j.fct.2007.03.008 | title = Variability and uncertainty assessment of patulin exposure for preschool children in Flanders | date = 2007 | last1 = Baert | first1 = Katleen | last2 = De Meulenaer | first2 = Bruno | last3 = Verdonck | first3 = Frederik | last4 = Huybrechts | first4 = Inge | last5 = De Henauw | first5 = Stefaan | last6 = Vanrolleghem | first6 = Peter A. | last7 = Debevere | first7 = Johan | last8 = Devlieghere | first8 = Frank | journal = Food and Chemical Toxicology | volume = 45 | issue = 9 | pages = 1745–1751 | pmid = 17459555 }}</ref> | |||
| ==References== | |||
| {{Reflist|2}} | |||
| == External links == | |||
| * {{Webarchive|url=https://web.archive.org/web/20171219144226/http://www.foodsafetywatch.org/factsheets/patulin/ |date=2017-12-19 }}, Food Safety Watch | |||
| ] | |||
| ] | |||
Latest revision as of 01:20, 18 May 2024
"Claviform" redirects here. For the botany and zoology term, see Glossary of entomology terms § clavate.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name 4-hydroxy-4H-furopyran-2(6H)-one | |||
| Other names
2-Hydroxy-3,7-dioxabicyclonona-5,9-dien-8-one Clairformin Claviform Expansine Clavacin Clavatin Expansin Gigantin Leucopin Patuline | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.215 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H6O4 | ||
| Molar mass | 154.12 g/mol | ||
| Appearance | Compact prisms | ||
| Density | 1.52 g/mL | ||
| Melting point | 110 °C (230 °F; 383 K) | ||
| Solubility in water | Soluble | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Patulin is an organic compound classified as a polyketide. It is named after the fungus from which it was isolated, Penicillium patulum. It is a white powder soluble in acidic water and in organic solvents. It is a lactone that is heat-stable, so it is not destroyed by pasteurization or thermal denaturation. However, stability following fermentation is lessened. It is a mycotoxin produced by a variety of molds, in particular, Aspergillus and Penicillium and Byssochlamys. Most commonly found in rotting apples, the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. Its presence is highly regulated.
Biosynthesis, synthesis, and reactivity
Patulin is biosynthesized from 6-methylsalicylic acid via multiple chemical transformations.
Isoepoxydon dehydrogenase (IDH) is an important enzyme in the multi-step biosynthesis of patulin. Its gene is present in other fungi that may potentially produce the toxin. It is reactive with sulfur dioxide, so antioxidant and antimicrobial agents may be useful to destroy it. Levels of nitrogen, manganese, and pH as well as abundance of necessary enzymes regulate the biosynthetic pathway of patulin.
Uses
Patulin was originally used as an antibiotic against Gram-positive and Gram-negative bacteria, but after several toxicity reports, it is no longer used for that purpose. Isolated by Nancy Atkinson in 1943, it was specifically trialed to be used against the common cold. Patulin is used as a potassium-uptake inhibitor in laboratory applications. Kashif Jilani and co-workers reported that patulin stimulates suicidal erythrocyte death under physiological concentrations.
Sources of exposure
Frequently, patulin is found in apples and apple products such as juices, jams, and ciders. It has also been detected in other fruits including cherries, blueberries, plums, bananas, strawberries, and grapes. Fungal growth leading to patulin production is most common on damaged fruits. Patulin has also been detected in grains like barley, wheat, corn and their processed products as well as in shellfish. Dietary intake of patulin from apple juice has been estimated at between 0.03 and 0.26 μg per kg body weight per day in various age groups and populations. Content of patulin in apple juice is estimated to be less than 10–15 μg/L. A number of studies have looked into comparisons of organic vs conventional harvest of apples and levels of patulin contamination. For example, one study showed 0.9% of children drinking organic apple juice exceeded the tolerable daily intake (TDI) for patulin. A recent article described detection of patulin in marine strains of Penicillium, indicating a potential risk in shellfish consumption.
Toxicity
A subacute rodent NOAEL of 43 μg/kg body weight as well as genotoxicity studies were primarily the cause for setting limits for patulin exposure, although a range of other types of toxicity also exist.
While not a particularly potent toxin, patulin is genotoxic. Some theorize that it may be a carcinogen, although animal studies have remained inconclusive. Patulin has shown antimicrobial properties against some microorganisms. Several countries have instituted patulin restrictions in apple products. The World Health Organization recommends a maximum concentration of 50 μg/L in apple juice. In the European Union, the limit is also set at 50 micrograms per kilogram (μg/kg) in apple juice and cider, at 25 μg/kg in solid apple products, and at 10 μg/kg in products for infants and young children. These limits came into force on 1 November 2003.
Acute
Patulin is toxic primarily through affinity to sulfhydryl groups (SH), which results in inhibition of enzymes. Oral LD50 in rodent models have ranged between 20 and 100 mg/kg. In poultry, the oral LD50 range was reported between 50 and 170 mg/kg. Other routes of exposure are more toxic, yet less likely to occur. Major acute toxicity findings include gastrointestinal problems, neurotoxicity (i.e. convulsions), pulmonary congestion, and edema.
Subacute
Studies in rats showed decreased weight, and gastric, intestinal, and renal function changes, while repetitive doses lead to neurotoxicity. Reproductive toxicity in males was also reported. A NOAEL in rodents was observed at 43 μg/kg body weight.
Genotoxicity
WHO concluded that patulin is genotoxic based on variable genotoxicity data, however it is considered a group 3 carcinogen by the International Agency for Research on Cancer (IARC) since data was inconclusive.
Reproduction studies
Patulin decreased sperm count and altered sperm morphology in the rat. Also, it resulted in abortion of F1 litters in rats and mice after i.p. injection. Embryotoxicity and teratogenicity were also reported in chick eggs.
Immunotoxicity
Patulin was found to be immunotoxic in a number of animal and even human studies. Reduced cytokine secretion, oxidative burst in macrophages, increased splenic T lymphocytes, and increased neutrophil numbers are a few endpoints noticed. However, dietary relevant exposure would not be likely to alter immune response.
Human health
Although there are only very few reported cases and epidemiological data, the FDA has set an action limit of 50 ppb in cider due to its potential carcinogenicity and other reported adverse effects. In humans, it was tested as an antiviral intranasally for use against the common cold with few significant adverse effects, yet also had negligible or no beneficial effect.
Risk management and regulations
Patulin exposure can be successfully managed by following good agricultural practices such as removing mold, washing, and not using rotten or damaged apples for baking, canning, or juice production.
US
The provisional tolerable daily intake (PTDI) for patulin was set at 0.43 μg/kg body weight by the FDA based on a NOAEL of 0.3 mg/kg body weight per week. Monte Carlo analysis was done on apple juice to compare exposure and the PTDI. Without controls or an action limit, the 90th percentile of consumers would not be above the PTDI. However, the concentration in children 1–2 years old would be three times as high as the PDTI, hence an action limit of 50 μg/kg.
WHO
The World Health Organization recommends a maximum concentration of 50 μg/L in apple juice.
EU
The European Union (EU) has set a maximum limit of 50 μg/kg on fruit juices and drinks, while solid apple products have a limit of 25 μg/kg. For certain foods intended for infants, an even lower limit of 10 μg/kg is observed.
To test for patulin contamination, a variety of methods and sample preparation methods have been employed, including thin layer chromatography (TLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), and capillary electrophoresis.
References
- ^ Merck Index, 11th Edition, 7002
- ^ Patulin sigmaaldrich.com
- ^ "Patulin in Apple Juice, Apple Juice Concentrates and Apple Juice Products". www.fda.gov. Archived from the original on 2013-08-15.
- Puel, Olivier; Galtier, Pierre; Oswald, Isabelle (2010). "Biosynthesis and Toxicological Effects of Patulin". Toxins. 2 (4): 613–631. doi:10.3390/toxins2040613. PMC 3153204. PMID 22069602.
- ^ Puel, Olivier; Galtier, Pierre; Oswald, Isabelle P. (5 April 2010). "Biosynthesis and Toxicological Effects of Patulin". Toxins. 2 (4): 613–631. doi:10.3390/toxins2040613. PMC 3153204. PMID 22069602.
- ^ Llewellyn, G.C; McCay, J.A; Brown, R.D; Musgrove, D.L; Butterworth, L.F; Munson, A.E; White, K.L (1998). "Immunological evaluation of the mycotoxin patulin in female b6C3F1 mice". Food and Chemical Toxicology. 36 (12): 1107–1115. doi:10.1016/s0278-6915(98)00084-2. PMID 9862653.
- ^ Medical Research Council. Clinical trial of patulin in the common cold. Lancet1944; ii: 373-5.
- Lupescu, A; Jilani, K; Zbidah, M; Lang, F (2013). "Patulin-induced suicidal erythrocyte death". Cellular Physiology and Biochemistry. 32 (2): 291–9. doi:10.1159/000354437. PMID 23942252.
- ^ "Patulin". Archived from the original on 2013-10-18. Retrieved 2013-11-25.
- ^ Pouchous et al. Shellfish
- ^ Wouters, FA, and Speijers, GJA. JECFA Monograph on Patulin. World Health Organization Food Additives Series 35 (http://www.inchem.org/documents/jecfa/jecmono/v26je10.htm)
- Pique, E., et al. Occurrence of patulin in organic and conventional apple juice. Risk Assessment. Recent Advances in Pharmaceutical Sciences, III, 2013: 131–144.
- Piemontese, L.; Solfrizzo, M.; Visconti, A. (2005-05-01). "Occurrence of patulin in conventional and organic fruit products in Italy and subsequent exposure assessment". Food Additives and Contaminants. 22 (5): 437–442. doi:10.1080/02652030500073550. ISSN 0265-203X. PMID 16019815. S2CID 31155096.
- Piqué, E; Vargas-Murga, L; Gómez-Catalán, J; Lapuente, Jd; Llobet, JM (October 2013). "Occurrence of patulin in organic and conventional apple-based food marketed in Catalonia and exposure assessment". Food and Chemical Toxicology. 60: 199–204. doi:10.1016/j.fct.2013.07.052. PMID 23900007.
- Beark et al 2007
- "Patulin: a Mycotoxin in Apples". Perishables Handling Quarterly (91): 5. August 1997
- ^ "Foodborne hazards (World Health Organization". Retrieved 2007-01-22.
- Patulin information leaf from Fermentek
- Selmanoglu, G (2006). "Evaluation of the reproductive toxicity of patulin in growing male rats". Food Chem. Toxicol. 44 (12): 2019–2024. doi:10.1016/j.fct.2006.06.022. PMID 16905234.
- Baert, Katleen; De Meulenaer, Bruno; Verdonck, Frederik; Huybrechts, Inge; De Henauw, Stefaan; Vanrolleghem, Peter A.; Debevere, Johan; Devlieghere, Frank (2007). "Variability and uncertainty assessment of patulin exposure for preschool children in Flanders". Food and Chemical Toxicology. 45 (9): 1745–1751. doi:10.1016/j.fct.2007.03.008. PMID 17459555.
External links
- Patulin Archived 2017-12-19 at the Wayback Machine, Food Safety Watch