| Revision as of 12:34, 10 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,084 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII').← Previous edit | Latest revision as of 00:45, 1 July 2022 edit undoCitation bot (talk | contribs)Bots5,460,407 edits Add: s2cid. | Use this bot. Report bugs. | Suggested by Abductive | Category:Multiple chemicals in an infobox that need indexing | #UCB_Category 1380/1863 | ||
| (19 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
| {{distinguish|phosphoramidon}} | {{distinguish|phosphoramidon}} | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | |ImageFile=Phosphamidon. |
||
| ⚫ | | verifiedrevid = 444050677 | ||
| ⚫ | |ImageSize= |
||
| ⚫ | | ImageFile =(E,Z)-Phosphamidon Structural Formulae V.1.svg | ||
| ⚫ | |IUPACName=(''E''/''Z'')- dimethyl phosphate | ||
| ⚫ | | ImageSize =360px | ||
| ⚫ | |OtherNames=Dimecron | ||
| ⚫ | | IUPACName =(''E''/''Z'')- dimethyl phosphate | ||
| ⚫ | | OtherNames =Dimecron | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | |
| CASNo = 13171-21-6 | ||
| | CASOther = <br>297-99-4 (''E'')<br>23783-98-4 (''Z'') | |||
| | CASNo2_Ref = {{cascite|correct|CAS}} | |||
| | PubChem=25750 | |||
| | CASNo2 = 297-99-4 | |||
| | CASNo2_Comment = (''E'') | |||
| | CASNo3_Ref = {{cascite|correct|CAS}} | |||
| | CASNo3 = 23783-98-4 | |||
| | CASNo3_Comment = (''Z'') | |||
| | PubChem =25750 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C18689 | | KEGG = C18689 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | |
| UNII = 7H857A6N6H | ||
| ⚫ | | SMILES=CCN(CC)C(=O)C(=C(C)OP(=O)(OC)OC)Cl | ||
| | UNII1_Ref = {{fdacite|correct|FDA}} | |||
| | UNII1 = 54VR7A0BQD | |||
| | UNII1_Comment = (''E'') | |||
| | UNII2_Ref = {{fdacite|correct|FDA}} | |||
| | UNII2 = HQ7958Q90Z | |||
| | UNII2_Comment = (''Z'') | |||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | ChemSpiderID = 23990 | |||
| ⚫ | | SMILES = CCN(CC)C(=O)/C(=C(\C)/OP(=O)(OC)OC)/Cl | ||
| | InChI = 1/C10H19ClNO5P/c1-6-12(7-2)10(13)9(11)8(3)17-18(14,15-4)16-5/h6-7H2,1-5H3 | |||
| | InChIKey = RGCLLPNLLBQHPF-UHFFFAOYAA | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChI = 1S/C10H19ClNO5P/c1-6-12(7-2)10(13)9(11)8(3)17-18(14,15-4)16-5/h6-7H2,1-5H3 | |||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | StdInChIKey = RGCLLPNLLBQHPF-UHFFFAOYSA-N | |||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
| C=10|H=19|Cl=1|N=1|O=5|P=1 | ||
| | |
| Appearance = | ||
| | |
| Density =1.2132 g/cm<sup>3</sup><ref name=inchem>, ]</ref> | ||
| | MeltingPtC = 120 to 123 | |||
| | MeltingPt=120-123 °C<ref name=Jacques>{{cite journal | title = Toxicology and pharmacology of a new systemic phosphoric acid ester insecticide phosphamidon (2-chloro-2-diethylcarbamoyl-1-methylvinyl dimethyl phosphate) | author = Jacques, R.; Bein, H. J. | journal = Archiv fuer Toxikologie | year = 1960 | volume = 18 | pages = 316–330}}</ref> | |||
| | |
| MeltingPt_ref = <ref name=Jacques>{{cite journal | title = Toxicology and pharmacology of a new systemic phosphoric acid ester insecticide phosphamidon (2-chloro-2-diethylcarbamoyl-1-methylvinyl dimethyl phosphate) |author1=Jacques, R. |author2=Bein, H. J. | journal = Archiv für Toxikologie | year = 1960 | volume = 18 | pages = 316–330|doi=10.1007/BF02226232 |s2cid=6714997 }}</ref> | ||
| | BoilingPtC = 162 | |||
| | Solubility=Miscible | |||
| | BoilingPt_notes = (1.5 mmHg)<ref name=Bachmann>{{cite journal | title = Phosphamidon, a new phosphate ester with systemic action | author = Bachmann, Fritz | journal = Proc. Intern. Cong. Crop. Protection, 4th Congr., Hamburg |year = 1960 | volume = 2 | pages = P1153-1155}}</ref> | |||
| | Solubility =Miscible | |||
| }} | }} | ||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| | |
| MainHazards = | ||
| | |
| FlashPt = | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| | |
| LD50 = 13 mg/kg (mouse, oral)<ref name=Jacques/><br />6 mg/kg (mouse, IV)<ref name=Jacques/><br />20 mg/kg (rat, oral)<ref name=Jacques/><br />26 mg/kg (rat, subcut.)<ref name=Jacques/> | ||
| }} | }} | ||
| }} | }} | ||
| Line 36: | Line 61: | ||
| == Toxicity and regulation== | == Toxicity and regulation== | ||
| Phosphamidon is very highly toxic to mammals and is listed as WHO Hazard Class Ia.<ref name=inchem/> A harvester developed symptoms of moderately severe poisoning after working in a field that had been sprayed with the chemical 2 weeks earlier. He collapsed and exhibited significant depression of serum cholinesterase, but recovered completely within 2 days after successful treatment with atropine.<ref>S. Gitelson, J. T. Davidson, A. Werczberger. Phosphamidon poisoning. |
Phosphamidon is very highly toxic to mammals and is listed as WHO Hazard Class Ia.<ref name=inchem/> A harvester developed symptoms of moderately severe poisoning after working in a field that had been sprayed with the chemical 2 weeks earlier. He collapsed and exhibited significant depression of serum cholinesterase, but recovered completely within 2 days after successful treatment with atropine.<ref>S. Gitelson, J. T. Davidson, A. Werczberger. Phosphamidon poisoning. Br. J. Ind. Med. 22: 236-239, 1965.</ref> International trade of phosphamidon is covered by the ]. | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Insecticides}} | |||
| ⚫ | ] | ||
| {{Acetylcholine metabolism and transport modulators}} | |||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:45, 1 July 2022
Not to be confused with phosphoramidon.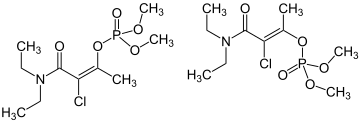 | |
| Names | |
|---|---|
| IUPAC name (E/Z)- dimethyl phosphate | |
| Other names Dimecron | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.818 |
| KEGG | |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H19ClNO5P |
| Molar mass | 299.69 g·mol |
| Density | 1.2132 g/cm |
| Melting point | 120 to 123 °C (248 to 253 °F; 393 to 396 K) |
| Boiling point | 162 °C (324 °F; 435 K) (1.5 mmHg) |
| Solubility in water | Miscible |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 13 mg/kg (mouse, oral) 6 mg/kg (mouse, IV) 20 mg/kg (rat, oral) 26 mg/kg (rat, subcut.) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Phosphamidon is an organophosphate insecticide first reported in 1960. It acts as a cholinesterase inhibitor.
The commercial product typically exists as a mixture of 70% (Z)-isomer and 30% (E)-isomer.
Toxicity and regulation
Phosphamidon is very highly toxic to mammals and is listed as WHO Hazard Class Ia. A harvester developed symptoms of moderately severe poisoning after working in a field that had been sprayed with the chemical 2 weeks earlier. He collapsed and exhibited significant depression of serum cholinesterase, but recovered completely within 2 days after successful treatment with atropine. International trade of phosphamidon is covered by the Rotterdam Convention.
References
- ^ Data Sheet on Pesticides No. 74: Phosphamidon, International Programme on Chemical Safety
- ^ Bachmann, Fritz (1960). "Phosphamidon, a new phosphate ester with systemic action". Proc. Intern. Cong. Crop. Protection, 4th Congr., Hamburg. 2: P1153-1155.
- ^ Jacques, R.; Bein, H. J. (1960). "Toxicology and pharmacology of a new systemic phosphoric acid ester insecticide phosphamidon (2-chloro-2-diethylcarbamoyl-1-methylvinyl dimethyl phosphate)". Archiv für Toxikologie. 18: 316–330. doi:10.1007/BF02226232. S2CID 6714997.
- S. Gitelson, J. T. Davidson, A. Werczberger. Phosphamidon poisoning. Br. J. Ind. Med. 22: 236-239, 1965.