| Revision as of 01:45, 7 September 2011 editHeadbomb (talk | contribs)Edit filter managers, Autopatrolled, Extended confirmed users, Page movers, File movers, New page reviewers, Pending changes reviewers, Rollbackers, Template editors455,093 editsm Various citation cleanup. using AWB← Previous edit | Latest revision as of 15:29, 30 December 2024 edit undoThe exergy conservationist (talk | contribs)23 edits →Properties: Page referred to ternary and quaternary alloys between BAs and GaAs. However, together these two binary alloys don't have enough different types of atoms to form a quaternary alloy. According to the linked reference, the quaternaries include indium (i.e., form BInGaAs).Tags: Mobile edit Mobile web edit | ||
| (193 intermediate revisions by 80 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | verifiedrevid = |
| verifiedrevid = 428779018 | ||
| | |
| Name = Boron arsenide | ||
| | |
| ImageFile = Boron-arsenide-unit-cell-1963-CM-3D-balls.png | ||
| | ImageCaption = | |||
| <!-- | ImageSize = 200px --> | |||
| |Section1={{Chembox Identifiers | |||
| | ImageName = BAs | |||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | ImageFile = B12As2_3D_side_view.jpg | |||
| | CASNo = 12005-69-5 | |||
| | ChemSpiderID = 8461243 | |||
| | ImageName = B<sub>12</sub>As<sub>2</sub> | |||
| | PubChem = 10285774 | |||
| | Section1 = {{Chembox Identifiers | |||
| | StdInChI=1S/AsB/c1-2 | |||
| | CASNo = 12005-69-5 | |||
| | StdInChIKey = DBKNIEBLJMAJHX-UHFFFAOYSA-N | |||
| | SMILES = # | |||
| }} | |||
| |Section2={{Chembox Properties | |||
| | Formula = BAs | |||
| | MolarMass = 85.733 g/mol<ref name=b92>{{RubberBible92nd|page=4.53}}</ref> | |||
| | Appearance =Brown cubic crystals<ref name=b92/> | |||
| | Density = 5.22 g/cm<sup>3</sup><ref name=b92/> | |||
| | Solubility = Insoluble | |||
| | MeltingPtC = 1100 | |||
| | ThermalConductivity = 1300 W/(m·K) (300 K) | |||
| | MeltingPt_notes = decomposes<ref name=b92/> | |||
| | BoilingPtC = | |||
| | BandGap = 1.82 eV | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | Structure_ref =<ref>{{cite journal|doi=10.1107/S0365110X58000827|title=New group III-group V compounds: BP and BAs|journal=Acta Crystallographica|volume=11|issue=4|pages=310|year=1958|last1=Perri|first1=J. A|last2=La Placa|first2=S|last3=Post|first3=B|doi-access=free|bibcode=1958AcCry..11..310P }}</ref> | |||
| | CrystalStruct = Cubic (]), ], No. 216 | |||
| | SpaceGroup = F{{overline|4}}3m | |||
| | LattConst_a = 0.4777 nm | |||
| | LattConst_b = | |||
| | LattConst_c = | |||
| | LattConst_alpha = | |||
| | LattConst_beta = | |||
| | LattConst_gamma = | |||
| | UnitCellVolume = | |||
| | UnitCellFormulas = 4 | |||
| | Coordination = | |||
| | MolShape = | |||
| | OrbitalHybridisation = | |||
| | Dipole = | |||
| }} | }} | ||
| | |
|Section4={{Chembox Thermochemistry | ||
| | DeltaHf = | |||
| | Formula = BAs or B<sub>12</sub>As<sub>2</sub> | |||
| | Entropy = | |||
| | MolarMass = 85.733 g/mol | |||
| }} | |||
| | Appearance = | |||
| |Section8={{Chembox Related | |||
| | Density = 5.22 g/cm<sup>3</sup>, solid | |||
| | OtherAnions = ]<br />]<br />] | |||
| | Solubility = Insoluble | |||
| | OtherCations = ]<br />]<br />] | |||
| | MeltingPtC = 2027 | |||
| }} | |||
| | BoilingPtC = | |||
| }} | |||
| | BandGap = 1.50 eV(BAs); 3.47 eV(B<sub>12</sub>As<sub>2</sub>) | |||
| {{Chembox | |||
| }} | |||
| | verifiedrevid = | |||
| | Section4 = {{Chembox Thermochemistry | |||
| | Name = Boron subarsenide | |||
| | DeltaHf = | |||
| | ImageFile = B12As2_3D_side_view.jpg | |||
| | Entropy = | |||
| | ImageCaption = | |||
| }} | |||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | EUClass = N/A | |||
| | CASNo = 12005-70-8 | |||
| | RPhrases = | |||
| | ChemSpiderID = | |||
| | SPhrases = | |||
| | StdInChI=1S/2AsH2.B12/c;;1-2-3(1)5(1)6(1)4(1,2)8(2)7(2,3)9(3,5)11(5,6)10(4,6,8)12(7,8,9)11/h2*1H2; | |||
| }} | |||
| | StdInChIKey=RGSVMMFVXFQAMT-UHFFFAOYSA-N | |||
| | Section8 = {{Chembox Related | |||
| | SMILES = 12345671892%10%118%12%13%10%14%15%16%17%1835(6%16%19%12%14%1779%13%19)4%11%15%18.. | |||
| | OtherAnions = ]<br />]<br />] | |||
| }} | |||
| | OtherCations = ]<br />]<br />] | |||
| |Section2={{Chembox Properties | |||
| | Formula = B<sub>12</sub>As<sub>2</sub> | |||
| | MolarMass = 279.58 g/mol | |||
| | Appearance = | |||
| | Density = 3.56 g/cm<sup>3</sup><ref>Villars, Pierre (ed.) in ''Inorganic Solid Phases'', Springer, Heidelberg (ed.) | |||
| SpringerMaterials</ref> | |||
| | Solubility = Insoluble | |||
| | MeltingPtC = | |||
| | BoilingPtC = | |||
| | BandGap = 3.47 eV | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | Structure_ref =<ref>{{cite journal|doi=10.1557/PROC-97-145|title=Crystal Structure Refinements of Rhombohedral Symmetry Materials Containing Boron-Rich Icosahedra|journal=MRS Proceedings|volume=97|year=2011|last1=Morosin|first1=B|last2=Aselage|first2=T. L|last3=Feigelson|first3=R. S}}</ref> | |||
| | CrystalStruct = ], ], No. 166 | |||
| | SpaceGroup = R{{overline|3}}m | |||
| | LattConst_a = 0.6149 nm | |||
| | LattConst_b = 0.6149 nm | |||
| | LattConst_c = 1.1914 nm | |||
| | LattConst_alpha = 90 | |||
| | LattConst_beta = 90 | |||
| | LattConst_gamma = 120 | |||
| | UnitCellVolume = | |||
| | UnitCellFormulas = 6 | |||
| | Coordination = | |||
| | MolShape = | |||
| | OrbitalHybridisation = | |||
| | Dipole = | |||
| }} | }} | ||
| |Section8={{Chembox Related | |||
| | OtherAnions = ] | |||
| | OtherCations = | |||
| }} | |||
| }} | }} | ||
| '''Boron arsenide''' (or '''Arsenic boride''') is a chemical compound involving ] and ], usually with a ] BAs. Other boron arsenide compounds are known, such as the subarsenide {{chem2|B12As2}}. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects. | |||
| ==Properties== | |||
| '''Boron arsenide''' is a chemical compound of ] and ]. It is a cubic (]) ] with a ] of 0.4777 nm and an ] of roughly 1.5 eV. It can be alloyed with ]. | |||
| BAs is a cubic (]) ] in the ] family with a ] of 0.4777 nm and an ] of 1.82 eV. Cubic BAs is reported to decompose to the subarsenide B<sub>12</sub>As<sub>2</sub> at temperatures above 920 °C.<ref>{{cite journal|doi=10.1149/1.2401826|title=Preparation and Properties of Boron Arsenide Films|journal=Journal of the Electrochemical Society|volume=121|issue=3|pages=412|year=1974|last1=Chu|first1=T. L|last2=Hyslop|first2=A. E|bibcode=1974JElS..121..412C}}</ref> Boron arsenide has a melting point of 2076 °C. The thermal conductivity of BAs is exceptionally high, recently measured in single-crystal BAs to be around 1300 W/(m·K) at room temperature, making it the highest among all metals and semiconductors.<ref>{{cite journal|doi=10.1126/science.aat5522|title=Experimental observation of high thermal conductivity in boron arsenide|journal=Science|year = 2018|volume=361|issue=6402|pages=575–578|last1=Kang|first1=J.|last2=Li|first2=M.|last3=Wu|first3=H.|last4=Nguyen|first4=H.|last5=Hu|first5=Y.|pmid=29976798|doi-access=free|bibcode=2018Sci...361..575K}}</ref> | |||
| The basic physical properties of cubic BAs have been experimentally measured:<ref>{{cite journal|doi=10.1063/1.5116025|title=Basic physical properties of cubic boron arsenide|journal=Applied Physics Letters|year = 2019|volume=115|issue=12|pages=122103|arxiv=1911.11281 |last1=Kang |first1=Joon Sang |last2=Li |first2=Man |last3=Wu |first3=Huan |last4=Nguyen |first4=Huuduy |last5=Hu |first5=Yongjie |bibcode=2019ApPhL.115l2103K }}</ref> Band gap (1.82 eV), optical refractive index (3.29 at wavelength 657 nm), elastic modulus (326 GPa), shear modulus, Poisson's ratio, thermal expansion coefficient (3.85×10<sup>−6</sup>/K), and heat capacity. It can be alloyed with ] to produce ternary and with ] to form quaternary semiconductors.<ref name="APL2000">{{cite journal|doi=10.1063/1.126058|title=BGaInAs alloys lattice matched to GaAs|journal=Applied Physics Letters|volume=76|issue=11|pages=1443|year=2000|last1=Geisz|first1=J. F|last2=Friedman|first2=D. J|last3=Olson|first3=J. M|last4=Kurtz|first4=Sarah R|author4-link= Sarah Kurtz |last5=Reedy|first5=R. C|last6=Swartzlander|first6=A. B|last7=Keyes|first7=B. M|last8=Norman|first8=A. G|bibcode=2000ApPhL..76.1443G}}</ref> | |||
| Boron arsenide also occurs as an ] boride, B<sub>12</sub>As<sub>2</sub>.<ref>http://spectra.phy.bris.ac.uk/research_semiconductor.asp</ref> | |||
| It belongs to ''R-3m'' space group with a rhombohedral structure based on clusters of boron atoms and two-atom As-As chains. It's a wide bandgap semiconductor (3.47 eV) with the extraordinary ability to “self-heal” radiation damage. This form can be grown on substrates such as ]. | |||
| BAs has high electron and hole mobility, >1000 cm<sup>2</sup>/V/second, unlike silicon which has high electron mobility, but low hole mobility.<ref>{{Cite journal |last1=Shin |first1=Jungwoo |last2=Gamage |first2=Geethal Amila |last3=Ding |first3=Zhiwei |last4=Chen |first4=Ke |last5=Tian |first5=Fei |last6=Qian |first6=Xin |last7=Zhou |first7=Jiawei |last8=Lee |first8=Hwijong |last9=Zhou |first9=Jianshi |last10=Shi |first10=Li |last11=Nguyen |first11=Thanh |date=2022-07-22 |title=High ambipolar mobility in cubic boron arsenide |url=https://www.science.org/doi/10.1126/science.abn4290 |journal=Science |language=en |volume=377 |issue=6604 |pages=437–440 |doi=10.1126/science.abn4290 |pmid=35862526 |bibcode=2022Sci...377..437S |s2cid=250952849 |issn=0036-8075}}</ref> | |||
| In 2023, a study in journal ] reported that subjected to high pressure BAs decrease its thermal conductivity contrary to the typical increase seen in most materials.<ref>{{Cite news |date=2023-01-27 |title=Surprising heat transfer behaviour seen in new semiconductor under pressure |url=https://physicsworld.com/surprising-heat-transfer-behaviour-seen-in-new-semiconductor-under-pressure/ |access-date=2023-01-30 |website=Physics World |language=en-GB}}</ref><ref>{{Cite journal |last1=Li |first1=Suixuan |last2=Qin |first2=Zihao |last3=Wu |first3=Huan |last4=Li |first4=Man |last5=Kunz |first5=Martin |last6=Alatas |first6=Ahmet |last7=Kavner |first7=Abby |last8=Hu |first8=Yongjie |date=23 November 2022 |title=Anomalous thermal transport under high pressure in boron arsenide |url=https://www.nature.com/articles/s41586-022-05381-x |journal=] |language=en |volume=612 |issue=7940 |pages=459–464 |doi=10.1038/s41586-022-05381-x |pmid=36418403 |bibcode=2022Natur.612..459L |s2cid=253838186 |issn=1476-4687}}</ref><ref>{{cite magazine |last1=Remmel |first1=Ariana |title=Boron arsenide breaks the rules under pressure |magazine=] |date=2 January 2023 |volume=101 |issue=1 |page=6 |doi=10.1021/cen-10101-scicon3 |url=https://cen.acs.org/materials/semiconductor-breaks-rules-physics-under/100/web/2022/12 |access-date=2 April 2023}}</ref> | |||
| ==Boron subarsenide== | |||
| Boron arsenide also occurs as subarsenides, including the ] boride {{chem2|B12As2}}. It belongs to ''R{{overline|3}}m'' ] with a ] structure based on clusters of boron atoms and two-atom As–As chains. It is a wide-bandgap semiconductor (3.47 eV) with the extraordinary ability to "self-heal" radiation damage.<ref>{{cite journal|doi=10.1103/PhysRevB.51.11270|pmid=9977852|title=Defect clustering and self-healing of electron-irradiated boron-rich solids|journal=Physical Review B|volume=51|issue=17|pages=11270–11274|year=1995|last1=Carrard|first1=M|last2=Emin|first2=D|last3=Zuppiroli|first3=L|bibcode=1995PhRvB..5111270C}}</ref> This form can be grown on ]s such as ].<ref>{{cite journal | doi = 10.1063/1.2945635 | last1 = Chen | first1 = H. | year=2008 | last2 = Wang | first2 = G. | last3 = Dudley | first3 = M. | last4 = Xu | first4 = Z. | last5 = Edgar | first5 = J. H. | last6 = Batten | first6 = T. | last7 = Kuball | first7 = M. | last8 = Zhang | first8 = L. | last9 = Zhu | first9 = Y. | title = Single-Crystalline B<sub>12</sub>As<sub>2</sub> on ''m''-plane (1{{overline|1}}00) 15R-SiC | journal = Applied Physics Letters | volume = 92 | issue = 23 | page = 231917 | bibcode = 2008ApPhL..92w1917C | hdl = 2097/2186 | hdl-access = free }}</ref> Another use for ] fabrication<ref name="APL2000" /><ref>Boone, J. L. and Vandoren, T. P. (1980) ''Boron arsenide thin film solar cell development, ''Final Report, Eagle-Picher Industries, Inc., Miami, OK. .</ref> was proposed, but it is not currently used for this purpose. | |||
| ==Applications== | ==Applications== | ||
| Boron arsenide is most attractive for use in electronics thermal management. Experimental integration with ] transistors to form GaN-BAs heterostructures has been demonstrated and shows better performance than the best GaN ] devices on silicon carbide or diamond substrates. Manufacturing BAs composites was developed as highly conducting and flexible thermal interfaces.<ref>{{cite journal |doi=10.1038/s41467-021-21531-7 |title=Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management |journal=Nature Communications |volume=12 |pages=1284 |year=2021 |last1=Cui |first1=Ying |last2=Qin |first2=Zihao |last3=Wu |first3=Huan |last4=Li |first4=Man |last5=Hu |first5=Yongjie |issue=1 |pmid=33627644 |pmc=7904764 |bibcode=2021NatCo..12.1284C }}.</ref> | |||
| ]s can be fabricated from boron arsenide. It's also an attractive choice for devices exposed to radiation which can severely | |||
| degrade the electrical properties of conventional semiconductors, causing devices to cease functioning. Among the particularly intriguing possible applications for B<sub>12</sub>As<sub>2</sub> are beta cells, devices capable of producing electrical energy by coupling a radioactive beta emitter to a semiconductor junction, another space electronics. | |||
| First-principles calculations have predicted that the ] of cubic BAs is remarkably high, over 2,200 W/(m·K) at room temperature, which is comparable to that of diamond and graphite.<ref>, Phys.org news (July 8, 2013)</ref> Subsequent measurements yielded a value of only 190 W/(m·K) due to the high density of defects.<ref>{{cite journal |doi=10.1063/1.4913441 |title=Experimental study of the proposed super-thermal-conductor: BAs |journal=Applied Physics Letters |volume=106 |issue=7 |pages=074105 |year=2015 |last1=Lv |first1=Bing |last2=Lan |first2=Yucheng |last3=Wang |first3=Xiqu |last4=Zhang |first4=Qian |last5=Hu |first5=Yongjie |last6=Jacobson |first6=Allan J |last7=Broido |first7=David |last8=Chen |first8=Gang |last9=Ren |first9=Zhifeng |last10=Chu |first10=Ching-Wu |bibcode=2015ApPhL.106g4105L |hdl=1721.1/117852 |osti=1387754 |s2cid=54074851 |url=https://dspace.mit.edu/bitstream/1721.1/117852/1/Lv2015APL_BAs.pdf |hdl-access=free}}</ref><ref>{{cite journal |last1=Zheng |first1=Qiang |last2=Polanco |first2=Carlos A. |last3=Du |first3=Mao-Hua |last4=Lindsay |first4=Lucas R. |last5=Chi |first5=Miaofang |author-link5=Miaofang Chi |last6=Yan |first6=Jiaqiang |last7=Sales |first7=Brian C. |date=6 September 2018 |title=Antisite Pairs Suppress the Thermal Conductivity of BAs |journal=Physical Review Letters |volume=121 |issue=10 |page=105901 |arxiv=1804.02381 |bibcode=2018PhRvL.121j5901Z |doi=10.1103/PhysRevLett.121.105901 |pmid=30240242 |s2cid=206316624}}</ref> More recent first-principles calculations incorporating four-phonon scattering predict a thermal conductivity of 1400 W/(m·K).<ref>{{cite journal |doi=10.1103/PhysRevB.96.161201 |title=Four-phonon scattering significantly reduces intrinsic thermal conductivity of solids |journal=Physical Review B |volume=96 |issue=16 |pages=161201 |year=2017 |last1=Feng |first1=Tianli |last2=Lindsay |first2=Lucas |last3=Ruan |first3=Xiulin |bibcode=2017PhRvB..96p1201F |doi-access=free}}</ref> Later, defect-free boron arsenide crystals have been experimentally realized and measured with an ultrahigh thermal conductivity of 1300 W/(m·K), consistent with theory predictions. Crystals with small density of defects have shown thermal conductivity of 900–1000 W/(m·K).<ref>{{cite journal |doi=10.1126/science.aat8982 |pmid=29976796 |title=High thermal conductivity in cubic boron arsenide crystals |journal=Science |volume=361 |issue=6402 |pages=579–581 |year=2018 |last1=Li |first1=Sheng |last2=Zheng |first2=Qiye |last3=Lv |first3=Yinchuan |last4=Liu |first4=Xiaoyuan |last5=Wang |first5=Xiqu |last6=Huang |first6=Pinshane Y. |last7=Cahill |first7=David G. |last8=Lv |first8=Bing |bibcode=2018Sci...361..579L |doi-access=free}}</ref><ref>{{cite journal |doi=10.1126/science.aat7932 |pmid=29976797 |title=Unusual high thermal conductivity in boron arsenide bulk crystals |journal=Science |volume=361 |issue=6402 |pages=582–585 |year=2018 |last1=Tian |first1=Fei |last2=Song |first2=Bai |last3=Chen |first3=Xi |last4=Ravichandran |first4=Navaneetha K |last5=Lv |first5=Yinchuan |last6=Chen |first6=Ke |last7=Sullivan |first7=Sean |last8=Kim |first8=Jaehyun |last9=Zhou |first9=Yuanyuan |last10=Liu |first10=Te-Huan |last11=Goni |first11=Miguel |last12=Ding |first12=Zhiwei |last13=Sun |first13=Jingying |last14=Gamage |first14=Geethal Amila Gamage Udalamatta |last15=Sun |first15=Haoran |last16=Ziyaee |first16=Hamidreza |last17=Huyan |first17=Shuyuan |last18=Deng |first18=Liangzi |last19=Zhou |first19=Jianshi |last20=Schmidt |first20=Aaron J |last21=Chen |first21=Shuo |last22=Chu |first22=Ching-Wu |last23=Huang |first23=Pinshane Y |last24=Broido |first24=David |last25=Shi |first25=Li |last26=Chen |first26=Gang |last27=Ren |first27=Zhifeng |bibcode=2018Sci...361..582T |doi-access=free}}</ref> | |||
| The cubic-shaped boron arsenide has been discovered to be better at conducting heat and electricity than ], as well as reportedly better than silicon at conducting both electrons and its positively charged counterpart, the "electron-hole."<ref>{{cite news |last=General |first=Ryan |title=Chinese MIT professor helps discover 'game changer' months after espionage charges |language=en |publisher=NextShark |date=18 August 2022 |url=https://www.yahoo.com/news/chinese-mit-professor-helps-discover-215437385.html |accessdate=19 August 2022 }}</ref> | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| *{{cite journal |doi=10.1063/1.2945635 |last=Chen |first=H. ''et al'' |authorlink= |coauthors= |year=2008 |last2=Wang |month= |first2=Guan |last3=Dudley |first3=Michael |last4=Xu |first4=Zhou |last5=Edgar |first5=J. H. |last6=Batten |first6=Tim |last7=Kuball |first7=Martin |last8=Zhang |first8=Lihua |last9=Zhu |first9=Yimei|title=Single-crystalline B<sub>12</sub>As<sub>2</sub> on m-plane (1-100)15R-SiC |journal=Applied Physics Letters |volume=92 |issue=23 |pages=231917–1--231917–3 }} | |||
| *{{cite journal |last=Hart |first=Gus L. W. |authorlink= |coauthors=Zunger, Alex |year=2000 |month= |title=Electronic structure of BAs and boride III-V alloys |journal=Physical Review B |volume=62 |issue=20 |pages=13522–13537 |doi=10.1103/PhysRevB.62.13522 |arxiv=cond-mat/0009063 |url= |accessdate= |quote= }} | |||
| *{{cite book |title=Boron Chemistry at the Millennium |last=King |first=R. Bruce |authorlink= |coauthors= |year=1999 |publisher=Elsevier |location=New York |isbn=0444720065 |pages= |url= }} | |||
| *{{cite journal |last=Ownby |first=P. D. |authorlink= |coauthors= |year=1975 |month= |title=Ordered Boron Arsenide |journal=Journal of the American Ceramic Society |volume=58 |issue=7–8 |pages=359–360 |doi=10.1111/j.1151-2916.1975.tb11514.x |url= |accessdate= |quote= }} | |||
| ==External links== | ==External links== | ||
| * 2020 paper by Malica and Dal Corso - | |||
| * thermophysical database entry | |||
| * | * | ||
| *{{cite book | title = Boron Chemistry at the Millennium | last = King | first = R. B. | year = 1999 | publisher = Elsevier | location = New York | isbn = 0-444-72006-5 }} | |||
| *{{cite journal | last = Ownby | first = P. D. | year = 1975 | title = Ordered Boron Arsenide | journal = Journal of the American Ceramic Society | volume = 58 | issue = 7–8 | pages = 359–360 | doi = 10.1111/j.1151-2916.1975.tb11514.x }} | |||
| * , Science | |||
| {{Arsenides}} | |||
| {{Boron compounds}} | {{Boron compounds}} | ||
| Line 62: | Line 133: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 15:29, 30 December 2024
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | BAs |
| Molar mass | 85.733 g/mol |
| Appearance | Brown cubic crystals |
| Density | 5.22 g/cm |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) decomposes |
| Solubility in water | Insoluble |
| Band gap | 1.82 eV |
| Thermal conductivity | 1300 W/(m·K) (300 K) |
| Structure | |
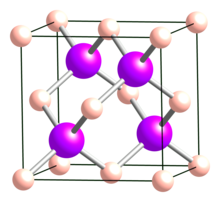
| Crystal structure | Cubic (sphalerite), cF8, No. 216 |
| Space group | F43m |
| Lattice constant | a = 0.4777 nm |
| Formula units (Z) | 4 |
| Related compounds | |
| Other anions | Boron nitride Boron phosphide Boron antimonide |
| Other cations | Aluminium arsenide Gallium arsenide Indium arsenide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | B12As2 |
| Molar mass | 279.58 g/mol |
| Density | 3.56 g/cm |
| Solubility in water | Insoluble |
| Band gap | 3.47 eV |
| Structure | |
| Crystal structure | Rhombohedral, hR42, No. 166 |
| Space group | R3m |
| Lattice constant | a = 0.6149 nm, b = 0.6149 nm, c = 1.1914 nmα = 90°, β = 90°, γ = 120° |
| Formula units (Z) | 6 |
| Related compounds | |
| Other anions | Boron suboxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Boron arsenide (or Arsenic boride) is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide B12As2. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects.
Properties
BAs is a cubic (sphalerite) semiconductor in the III-V family with a lattice constant of 0.4777 nm and an indirect band gap of 1.82 eV. Cubic BAs is reported to decompose to the subarsenide B12As2 at temperatures above 920 °C. Boron arsenide has a melting point of 2076 °C. The thermal conductivity of BAs is exceptionally high, recently measured in single-crystal BAs to be around 1300 W/(m·K) at room temperature, making it the highest among all metals and semiconductors.
The basic physical properties of cubic BAs have been experimentally measured: Band gap (1.82 eV), optical refractive index (3.29 at wavelength 657 nm), elastic modulus (326 GPa), shear modulus, Poisson's ratio, thermal expansion coefficient (3.85×10/K), and heat capacity. It can be alloyed with gallium arsenide to produce ternary and with indium gallium arsenide to form quaternary semiconductors.
BAs has high electron and hole mobility, >1000 cm/V/second, unlike silicon which has high electron mobility, but low hole mobility.
In 2023, a study in journal Nature reported that subjected to high pressure BAs decrease its thermal conductivity contrary to the typical increase seen in most materials.
Boron subarsenide
Boron arsenide also occurs as subarsenides, including the icosahedral boride B12As2. It belongs to R3m space group with a rhombohedral structure based on clusters of boron atoms and two-atom As–As chains. It is a wide-bandgap semiconductor (3.47 eV) with the extraordinary ability to "self-heal" radiation damage. This form can be grown on substrates such as silicon carbide. Another use for solar cell fabrication was proposed, but it is not currently used for this purpose.
Applications
Boron arsenide is most attractive for use in electronics thermal management. Experimental integration with gallium nitride transistors to form GaN-BAs heterostructures has been demonstrated and shows better performance than the best GaN HEMT devices on silicon carbide or diamond substrates. Manufacturing BAs composites was developed as highly conducting and flexible thermal interfaces.
First-principles calculations have predicted that the thermal conductivity of cubic BAs is remarkably high, over 2,200 W/(m·K) at room temperature, which is comparable to that of diamond and graphite. Subsequent measurements yielded a value of only 190 W/(m·K) due to the high density of defects. More recent first-principles calculations incorporating four-phonon scattering predict a thermal conductivity of 1400 W/(m·K). Later, defect-free boron arsenide crystals have been experimentally realized and measured with an ultrahigh thermal conductivity of 1300 W/(m·K), consistent with theory predictions. Crystals with small density of defects have shown thermal conductivity of 900–1000 W/(m·K).
The cubic-shaped boron arsenide has been discovered to be better at conducting heat and electricity than silicon, as well as reportedly better than silicon at conducting both electrons and its positively charged counterpart, the "electron-hole."
References
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.53. ISBN 1-4398-5511-0.
- Perri, J. A; La Placa, S; Post, B (1958). "New group III-group V compounds: BP and BAs". Acta Crystallographica. 11 (4): 310. Bibcode:1958AcCry..11..310P. doi:10.1107/S0365110X58000827.
- Villars, Pierre (ed.) "B12As2 (B6As) Crystal Structure" in Inorganic Solid Phases, Springer, Heidelberg (ed.) SpringerMaterials
- Morosin, B; Aselage, T. L; Feigelson, R. S (2011). "Crystal Structure Refinements of Rhombohedral Symmetry Materials Containing Boron-Rich Icosahedra". MRS Proceedings. 97. doi:10.1557/PROC-97-145.
- Chu, T. L; Hyslop, A. E (1974). "Preparation and Properties of Boron Arsenide Films". Journal of the Electrochemical Society. 121 (3): 412. Bibcode:1974JElS..121..412C. doi:10.1149/1.2401826.
- Kang, J.; Li, M.; Wu, H.; Nguyen, H.; Hu, Y. (2018). "Experimental observation of high thermal conductivity in boron arsenide". Science. 361 (6402): 575–578. Bibcode:2018Sci...361..575K. doi:10.1126/science.aat5522. PMID 29976798.
- Kang, Joon Sang; Li, Man; Wu, Huan; Nguyen, Huuduy; Hu, Yongjie (2019). "Basic physical properties of cubic boron arsenide". Applied Physics Letters. 115 (12): 122103. arXiv:1911.11281. Bibcode:2019ApPhL.115l2103K. doi:10.1063/1.5116025.
- ^ Geisz, J. F; Friedman, D. J; Olson, J. M; Kurtz, Sarah R; Reedy, R. C; Swartzlander, A. B; Keyes, B. M; Norman, A. G (2000). "BGaInAs alloys lattice matched to GaAs". Applied Physics Letters. 76 (11): 1443. Bibcode:2000ApPhL..76.1443G. doi:10.1063/1.126058.
- Shin, Jungwoo; Gamage, Geethal Amila; Ding, Zhiwei; Chen, Ke; Tian, Fei; Qian, Xin; Zhou, Jiawei; Lee, Hwijong; Zhou, Jianshi; Shi, Li; Nguyen, Thanh (2022-07-22). "High ambipolar mobility in cubic boron arsenide". Science. 377 (6604): 437–440. Bibcode:2022Sci...377..437S. doi:10.1126/science.abn4290. ISSN 0036-8075. PMID 35862526. S2CID 250952849.
- "Surprising heat transfer behaviour seen in new semiconductor under pressure". Physics World. 2023-01-27. Retrieved 2023-01-30.
- Li, Suixuan; Qin, Zihao; Wu, Huan; Li, Man; Kunz, Martin; Alatas, Ahmet; Kavner, Abby; Hu, Yongjie (23 November 2022). "Anomalous thermal transport under high pressure in boron arsenide". Nature. 612 (7940): 459–464. Bibcode:2022Natur.612..459L. doi:10.1038/s41586-022-05381-x. ISSN 1476-4687. PMID 36418403. S2CID 253838186.
- Remmel, Ariana (2 January 2023). "Boron arsenide breaks the rules under pressure". C&EN. Vol. 101, no. 1. p. 6. doi:10.1021/cen-10101-scicon3. Retrieved 2 April 2023.
- Carrard, M; Emin, D; Zuppiroli, L (1995). "Defect clustering and self-healing of electron-irradiated boron-rich solids". Physical Review B. 51 (17): 11270–11274. Bibcode:1995PhRvB..5111270C. doi:10.1103/PhysRevB.51.11270. PMID 9977852.
- Chen, H.; Wang, G.; Dudley, M.; Xu, Z.; Edgar, J. H.; Batten, T.; Kuball, M.; Zhang, L.; Zhu, Y. (2008). "Single-Crystalline B12As2 on m-plane (1100) 15R-SiC". Applied Physics Letters. 92 (23): 231917. Bibcode:2008ApPhL..92w1917C. doi:10.1063/1.2945635. hdl:2097/2186.
- Boone, J. L. and Vandoren, T. P. (1980) Boron arsenide thin film solar cell development, Final Report, Eagle-Picher Industries, Inc., Miami, OK. abstract.
- Cui, Ying; Qin, Zihao; Wu, Huan; Li, Man; Hu, Yongjie (2021). "Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management". Nature Communications. 12 (1): 1284. Bibcode:2021NatCo..12.1284C. doi:10.1038/s41467-021-21531-7. PMC 7904764. PMID 33627644..
- An unlikely competitor for diamond as the best thermal conductor, Phys.org news (July 8, 2013)
- Lv, Bing; Lan, Yucheng; Wang, Xiqu; Zhang, Qian; Hu, Yongjie; Jacobson, Allan J; Broido, David; Chen, Gang; Ren, Zhifeng; Chu, Ching-Wu (2015). "Experimental study of the proposed super-thermal-conductor: BAs" (PDF). Applied Physics Letters. 106 (7): 074105. Bibcode:2015ApPhL.106g4105L. doi:10.1063/1.4913441. hdl:1721.1/117852. OSTI 1387754. S2CID 54074851.
- Zheng, Qiang; Polanco, Carlos A.; Du, Mao-Hua; Lindsay, Lucas R.; Chi, Miaofang; Yan, Jiaqiang; Sales, Brian C. (6 September 2018). "Antisite Pairs Suppress the Thermal Conductivity of BAs". Physical Review Letters. 121 (10): 105901. arXiv:1804.02381. Bibcode:2018PhRvL.121j5901Z. doi:10.1103/PhysRevLett.121.105901. PMID 30240242. S2CID 206316624.
- Feng, Tianli; Lindsay, Lucas; Ruan, Xiulin (2017). "Four-phonon scattering significantly reduces intrinsic thermal conductivity of solids". Physical Review B. 96 (16): 161201. Bibcode:2017PhRvB..96p1201F. doi:10.1103/PhysRevB.96.161201.
- Li, Sheng; Zheng, Qiye; Lv, Yinchuan; Liu, Xiaoyuan; Wang, Xiqu; Huang, Pinshane Y.; Cahill, David G.; Lv, Bing (2018). "High thermal conductivity in cubic boron arsenide crystals". Science. 361 (6402): 579–581. Bibcode:2018Sci...361..579L. doi:10.1126/science.aat8982. PMID 29976796.
- Tian, Fei; Song, Bai; Chen, Xi; Ravichandran, Navaneetha K; Lv, Yinchuan; Chen, Ke; Sullivan, Sean; Kim, Jaehyun; Zhou, Yuanyuan; Liu, Te-Huan; Goni, Miguel; Ding, Zhiwei; Sun, Jingying; Gamage, Geethal Amila Gamage Udalamatta; Sun, Haoran; Ziyaee, Hamidreza; Huyan, Shuyuan; Deng, Liangzi; Zhou, Jianshi; Schmidt, Aaron J; Chen, Shuo; Chu, Ching-Wu; Huang, Pinshane Y; Broido, David; Shi, Li; Chen, Gang; Ren, Zhifeng (2018). "Unusual high thermal conductivity in boron arsenide bulk crystals". Science. 361 (6402): 582–585. Bibcode:2018Sci...361..582T. doi:10.1126/science.aat7932. PMID 29976797.
- General, Ryan (18 August 2022). "Chinese MIT professor helps discover 'game changer' months after espionage charges". NextShark. Retrieved 19 August 2022.
External links
- 2020 paper by Malica and Dal Corso - Temperature dependent elastic constants and thermodynamic properties of BAs: An ab initio investigation
- Matweb data
- King, R. B. (1999). Boron Chemistry at the Millennium. New York: Elsevier. ISBN 0-444-72006-5.
- Ownby, P. D. (1975). "Ordered Boron Arsenide". Journal of the American Ceramic Society. 58 (7–8): 359–360. doi:10.1111/j.1151-2916.1975.tb11514.x.
- High ambipolar mobility in cubic boron arsenide, Science
| Arsenides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binary arsenides |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ternary arsenides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quaternary arsenides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quinary arsenides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| See also | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boron compounds | |
|---|---|
| Boron pnictogenides | |
| Boron halides | |
| Acids | |
| Boranes | |
| Boron oxides and sulfides | |
| Carbides | |
| Organoboron compounds | |